SNP marker related to liver toxicity of platinum type chemotherapeutic medicines and applications thereof
A chemotherapeutic drug, liver toxicity technology, applied in the fields of genetic engineering and oncology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The collection of embodiment 1 sample and the arrangement of sample data
[0065] The inventor collected a large number of blood samples from primary NSCLC patients from April 2005 to January 2011 from the Affiliated Cancer Hospital of Nanjing Medical University and the First Affiliated Hospital of Nanjing Medical University. A total of 334 genome-wide microarray scan samples meeting the following criteria were selected:
[0066] (1) New-onset NSCLC patients admitted to the hospital for the first time and confirmed by histopathology or cytology, whose diagnosis is confirmed by at least two pathologists according to the standards issued by the World Health Organization;
[0067] (2) All indicators of liver function tests before the first chemotherapy were normal;
[0068] (3) Receive 2 to 6 cycles of platinum-based chemotherapy; exclude NSCLC patients who received radiotherapy before or during chemotherapy;
[0069] (4) Have detailed evaluation information on liver tox...
Embodiment 2
[0071] Whole Genome Scanning of SNP in Example 2 Peripheral Blood DNA
[0072] Affymetrix6.0 chip detection was performed on the peripheral blood DNA of the 334 eligible NSCLC patients receiving platinum-based chemotherapy to obtain relevant results. The specific steps are:
[0073] 1. Add hemolysis reagent (that is, lysate, 40 parts) to the blood cells stored in the 2ml cryopreservation tube. Dilute to 2000ml, the same below), invert and mix completely before transferring.
[0074] 2. Removal of red blood cells: Fill the 5ml centrifuge tube to 4ml with hemolysis reagent, mix by inverting, centrifuge at 4000rpm for 10 minutes, and discard the supernatant. Add 4ml of hemolysis reagent to the precipitate, invert and wash again, centrifuge at 4000rpm for 10 minutes, and discard the supernatant.
[0075] 3. Extract DNA: Add 1ml of extract solution to the precipitate (each 300ml contains 122.5ml0.2M sodium chloride, 14.4ml0.5M ethylenediaminetetraacetic acid, 15ml10% sodium dode...
Embodiment 3
[0084] Example 3 Using the risk scoring method to further analyze the liver toxicity of SNP and platinum-based chemotherapy drugs
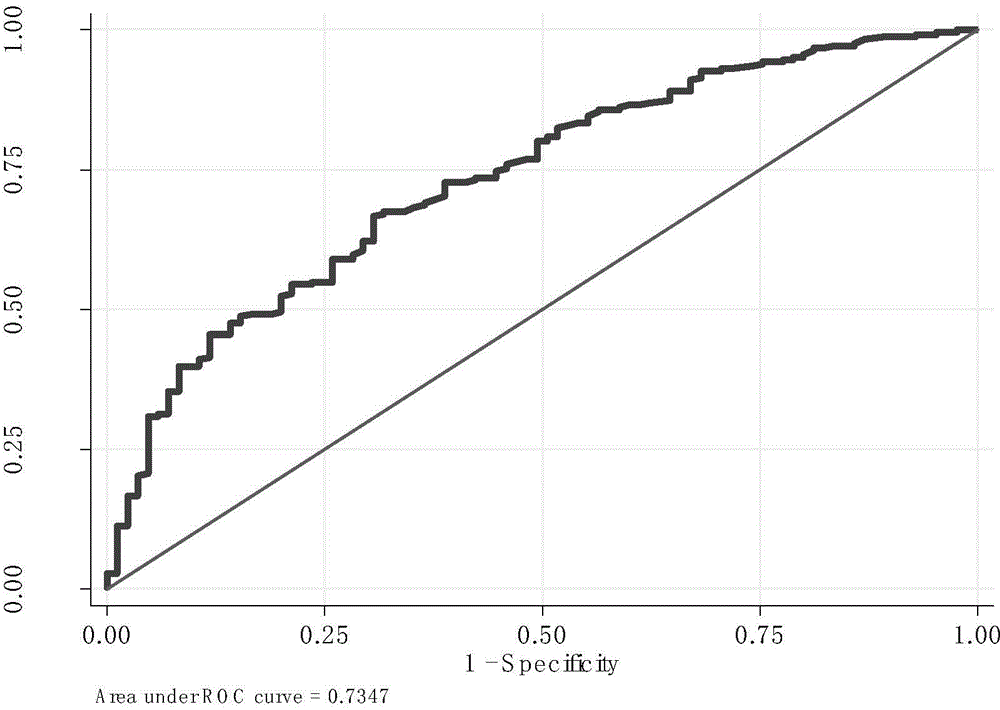
[0085] Based on the above results, the inventors selected the positively associated SNPs by comparing the genotype distribution frequencies of NSCLC patients with different degrees of liver toxicity, and used the regression coefficient of a single SNP in the whole genome scan sample as the weight to further obtain the risk score and draw ROC was used to evaluate the sensitivity and specificity of diagnosis, and then to evaluate the diagnostic ability of these SNPs for liver toxicity of platinum-based chemotherapy drugs. The combined analysis of 20 SNP markers found that these 20 SNPs separated NSCLC patients with hepatotoxicity from those without hepatotoxicity with a ROC of 73.5%, the sensitivity of the optimal cut-off point was 66.7%, and the specificity: 69.4% ( See figure 1 ).
[0086] 因此,本发明人证明了采用rs6681909、rs4140932、rs13131227、rs4446279、rs1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com