Triphenylamine pyridinium salt fluorescent molecule and preparation method thereof
A technology of triphenylamine pyridinium salt and fluorescent molecules, which is applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, and material analysis through optical means, and can solve problems such as difficult separation, high price, and low synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1. The preparation method of triphenylamine-pyridinium salt TPA-PyS
[0019] (1) Under nitrogen protection and anhydrous and oxygen-free conditions, triphenylamine iodide 4-[N,N-bis(4-iodophenyl)amino]benzaldehyde (TPA-I) (prepared according to the literature method, reference: Tetrahedron Letters, 2007, 48, 5878) and 4-vinylpyridine through palladium-catalyzed coupling reaction to prepare triphenylamine-pyridine compound 4-[N,N-bis(4-pyridylvinylphenyl)amino]benzaldehyde (TPA -Py), the synthetic route is as follows:
[0020]
[0021] The specific preparation method is: under nitrogen protection and anhydrous and oxygen-free conditions, dissolve 0.53g (1.0mmol) of 4-[N,N-bis(4-iodophenyl)amino]benzaldehyde in 10mL of anhydrous N,N- To dimethylacetamide were added 0.023 g (0.1 mmol) of palladium acetate, 0.061 g (0.2 mmol) of tris(o-methylphenyl)phosphine, and 0.60 g (2.8 mmol) of anhydrous potassium phosphate. Under magnetic stirring, 1.5 mL of 4-vinylpy...
Embodiment 2
[0033] Example 2. Red emission of triphenylamine-pyridinium salt TPA-PyS
[0034] The solid state of triphenylamine-pyridinium salt (TPA-PyS) emits red fluorescence, and the fluorescence emission peak is 656nm, which is located in the near-infrared region. attached figure 1 Fluorescence spectrum of the triphenylamine pyridinium salt TPA-PyS solid powder prepared in Example 1 of the present invention. The solid state emits red fluorescence under ultraviolet light in a dark room.
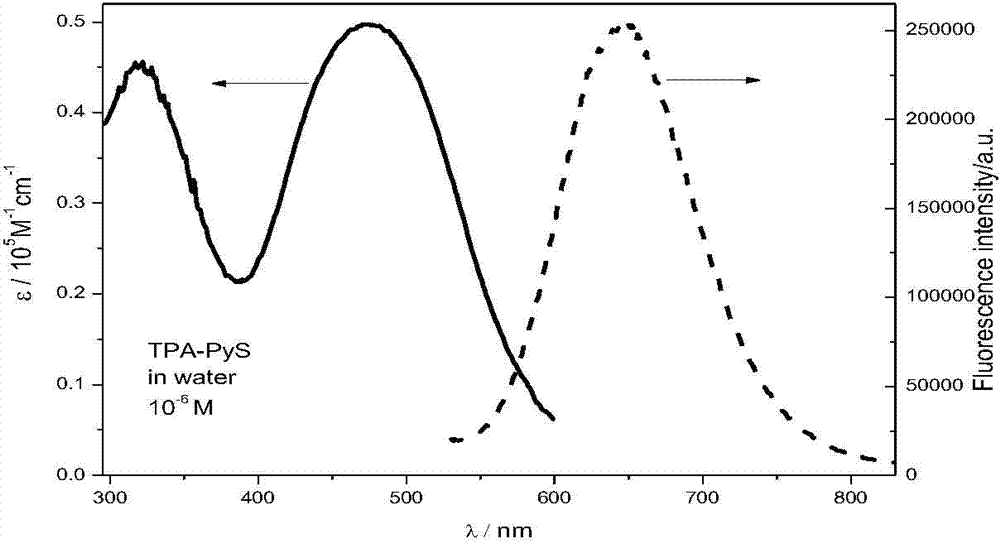
[0035] The pyridinium salt compound of the present invention is soluble in water, emits red light in water, the wavelength is 647nm, and figure 2 The ultraviolet-visible absorption spectrum and fluorescence spectrum of the triphenylamine pyridinium salt TPA-PyS prepared in Example 1 of the present invention in aqueous solution.
Embodiment 3
[0036] Example 3. Fluorescent probe properties of triphenylamine-pyridinium salt TPA-PyS
[0037] Under the physiological environment of pH 7.4, Na 2 HPO 4 -NaH 2 PO 4 As a buffer system, keep the probe molecule TPA-PyS concentration at 10 -6 M, BSA concentration gradually increased from 0 to 4.0×10 -6 M, the fluorescence spectrum of the measurement system. attached image 3 Fluorescence spectrum of the interaction between triphenylamine pyridinium salt TPA-PyS prepared in Example 1 of the present invention and bovine serum albumin. The near-infrared fluorescence intensity of TPA-PyS increased rapidly with the increase of bovine serum albumin BSA concentration, and the position of the fluorescence peak shifted significantly. Triphenylamine pyridinium salt TPA-PyS is soluble in water, has near-infrared emission fluorescence characteristics, and can be used as a near-infrared fluorescent probe for the detection of bovine serum albumin BSA.
[0038]The triphenylamine pyri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com