Preparation of nitrogen heterocyclic compound metal salt or its heterocyclic compound complex
A technology of nitrogen heterocyclic compounds and heterocyclic compounds, applied in lithium organic compounds, sodium organic compounds, organic chemistry, etc., can solve the problems of long time consumption, large solvent consumption, and difficulty in complete reaction balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
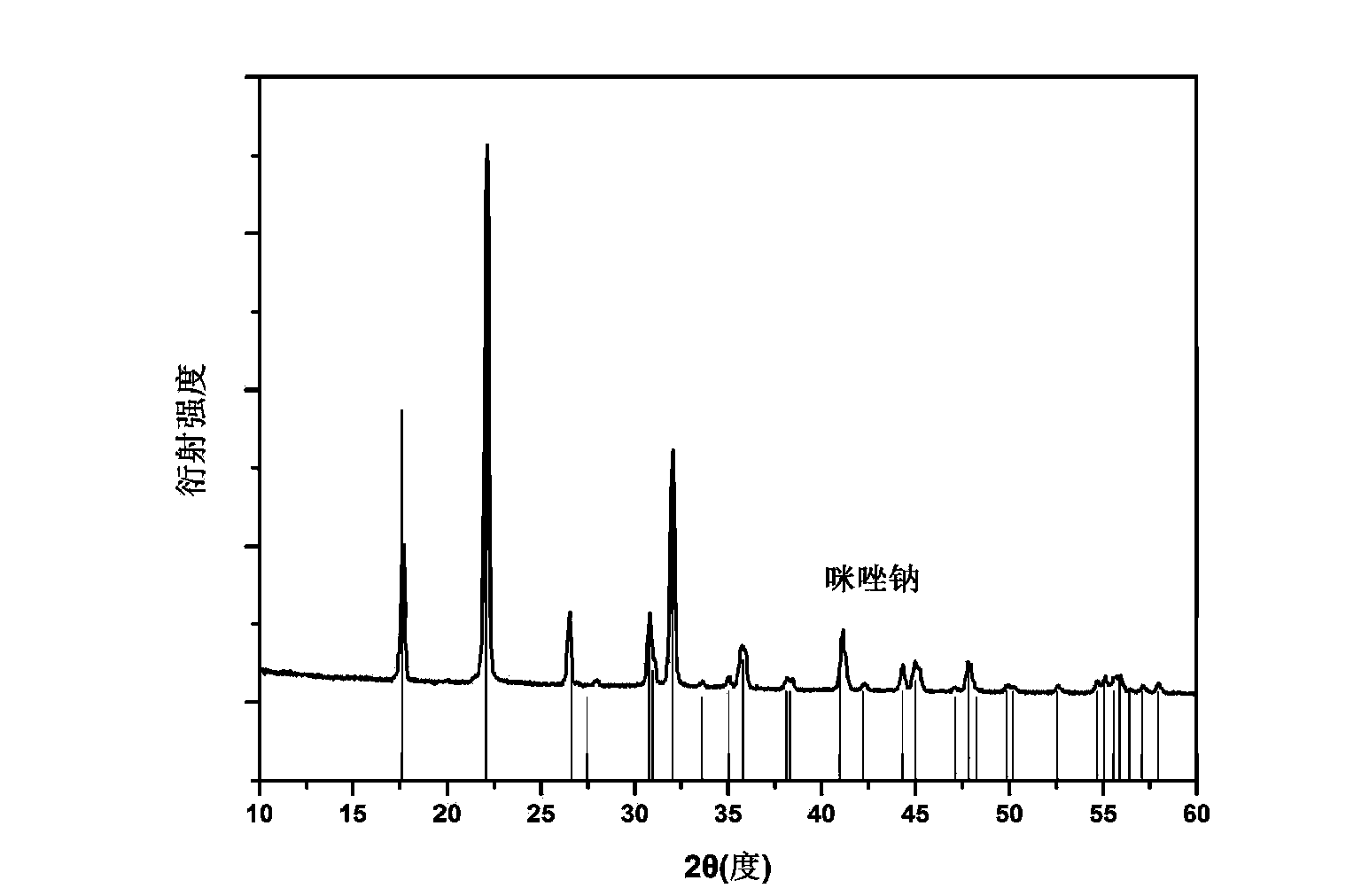
[0024] Embodiment 1: the preparation of imidazole sodium
[0025] In the glove box, weigh 687 mg of imidazole and 252 mg of sodium hydride in the same ball mill jar. Note that the two samples cannot be in contact at this time. After this ball mill jar is sealed, carefully transfer to the ball mill, under the condition of 50 ℃, under the rotating speed of 200rpm, ball mill for 5 hours. The degree of progress of the reaction can be realized by monitoring the pressure change in the ball mill tank. figure 1 It is the X-ray diffraction (XRD) spectrum of the prepared sample. It can be seen that it is consistent with the diffraction peak of imidazole sodium in the database, which proves that we have synthesized imidazole sodium and illustrates the feasibility of this preparation method.
Embodiment 2
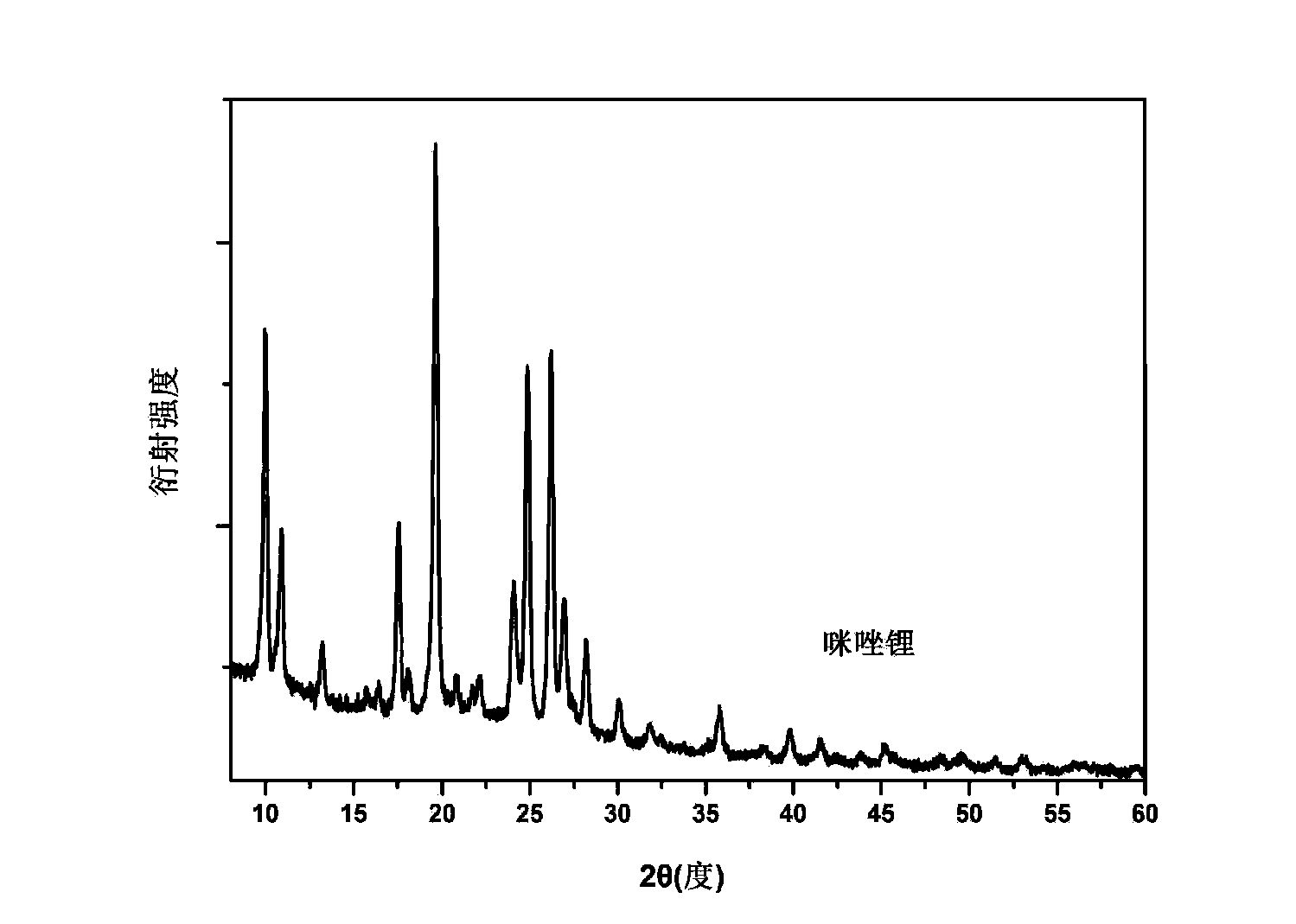
[0026] Embodiment 2: the preparation of imidazole lithium
[0027] In the glove box, weigh 687 mg of imidazole and 81 mg of lithium hydride in the same ball mill jar. Note that the two samples cannot be in contact at this time. After this ball mill jar is sealed, carefully transfer to the ball mill, under the condition of 50 ℃, under the rotating speed of 200rpm, ball mill for 20 hours. The degree of progress of the reaction can be realized by monitoring the pressure change in the ball mill tank. figure 2 For the X-ray diffraction (XRD) spectrum of the prepared sample, it can be seen that the diffraction peaks of imidazole and lithium hydride have disappeared, replaced by a new set of diffraction peaks, which proves that a new species has been formed, namely our The target product is lithium imidazole.
Embodiment 3
[0028] Embodiment 3: the preparation of pyrrole sodium, pyrrole lithium
[0029] In the glove box, weigh 252 mg of sodium hydride (or 81 mg of lithium hydride), and measure 0.5 ml of pyrrole in the same ball mill jar. Note that the two samples cannot be in contact at this time. After the ball mill jar was sealed, it was carefully transferred to a ball mill, and ball milled at 150 rpm for 5 hours at room temperature. The degree of progress of the reaction can be realized by monitoring the pressure change in the ball mill tank. image 3 X-ray diffraction (XRD) spectra of the prepared pyrrole sodium and pyrrole lithium powder samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com