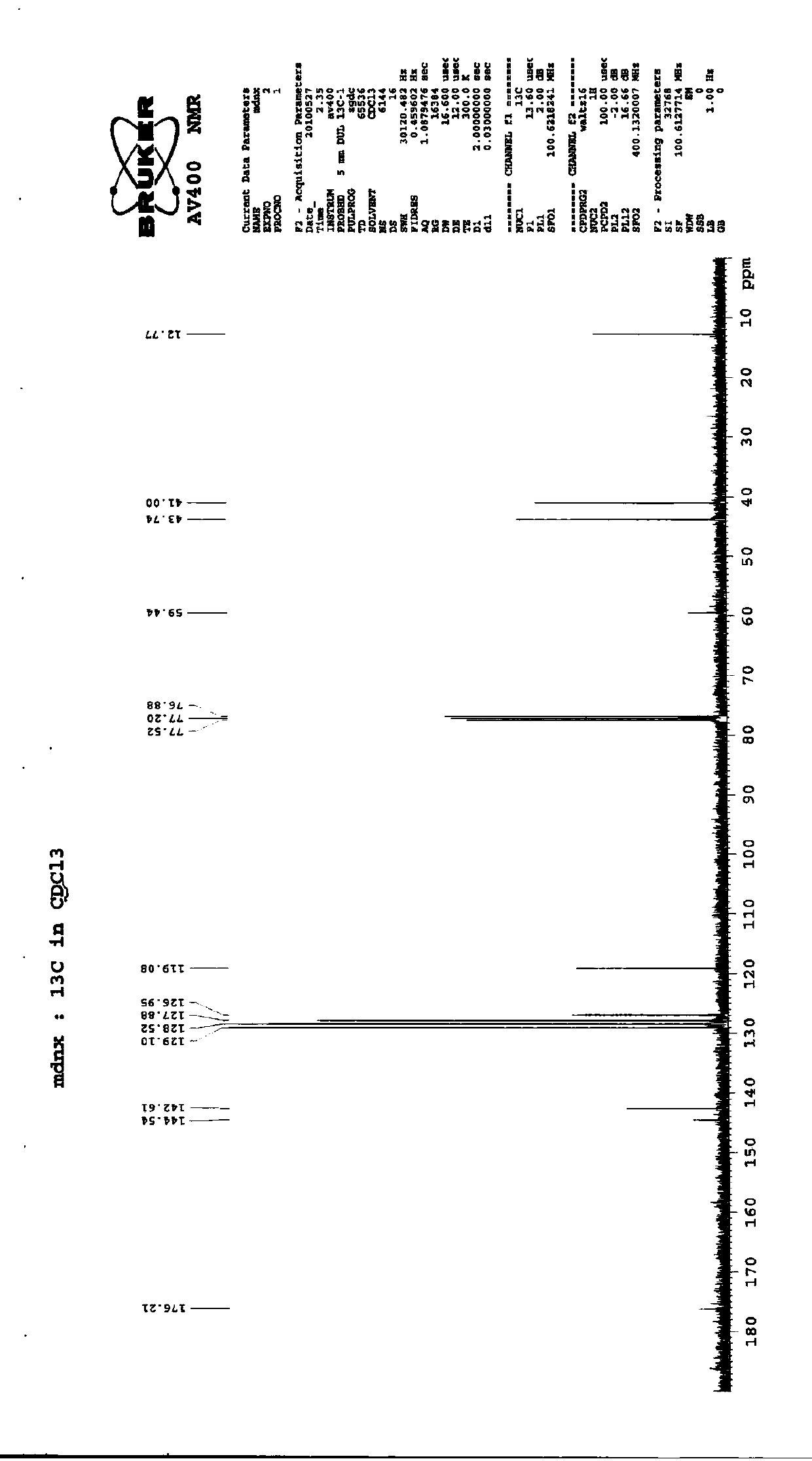
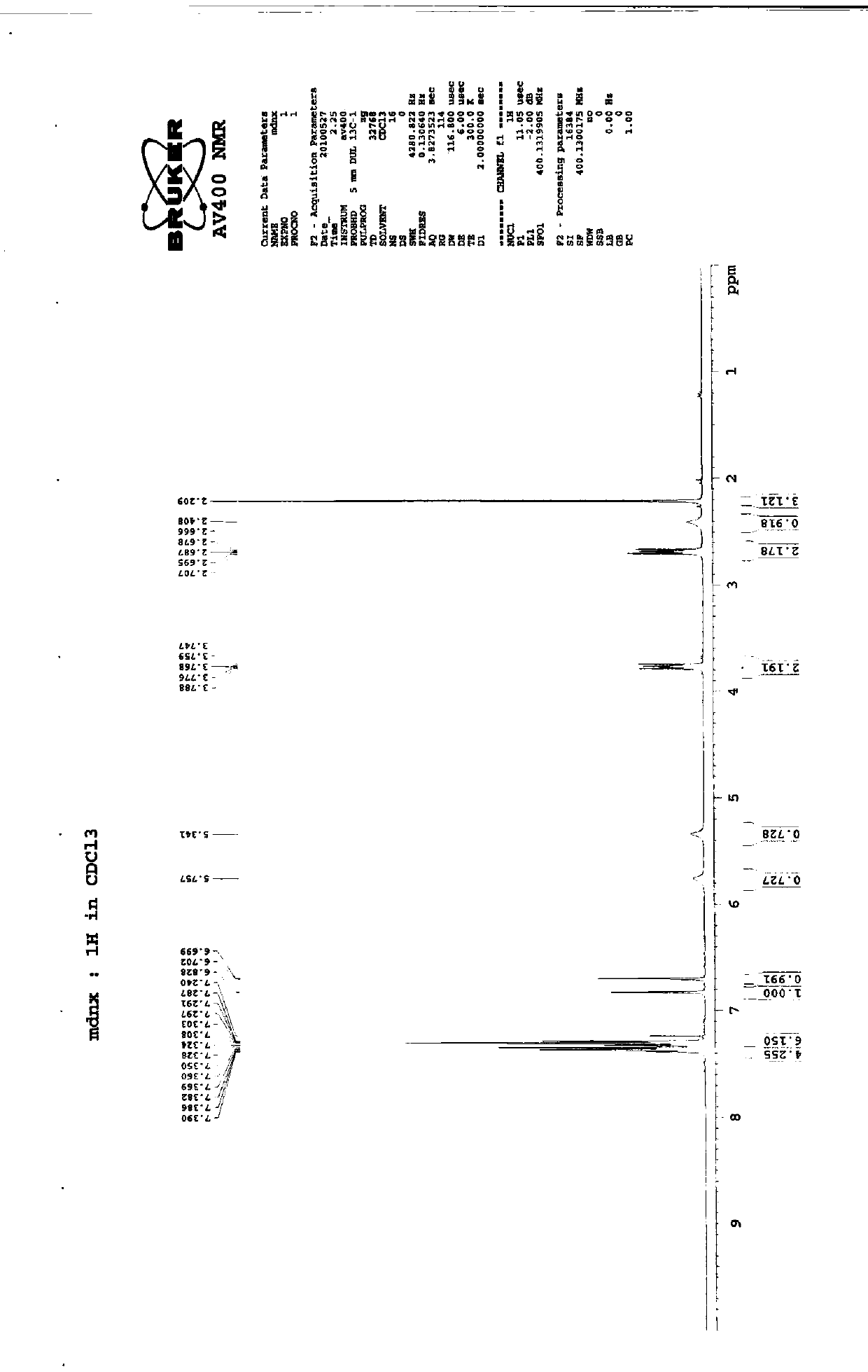
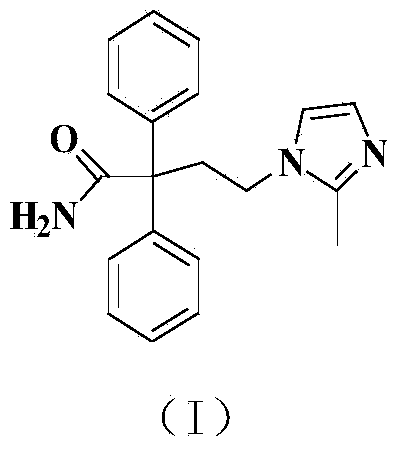
Preparation method of imidafenacin
A technology of imidazole and methylimidazole, which is applied in the field of preparation of midanacin, a new drug for the treatment of overactive bladder, can solve the problems of deflagration, many impurities, and complex components of the final product.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Preparation of 1-(2-bromoethyl)-2-methyl-1H-imidazole (5)
[0024] 1,2-Dibromoethane (50ml), 2-methylimidazole (2.5g, 30.5mmol), tetrabutylammonium bromide (TBAB) (0.5g) and K 2 CO 3 (3.6g) and KOH (4.6g) were sequentially added into a 100ml three-neck flask, stirred and heated to 50°C for 7h. After cooling to room temperature, the reaction solution was filtered, the filtrate was washed with saturated aqueous sodium bicarbonate solution, and dried over anhydrous sodium sulfate. Concentrate, add a mixed solvent of isopropyl ether and ethyl acetate (3:1) and stir to dissolve and crystallize to obtain 5.1 g of the product, with a yield of 88.5%, mp.79-80°C.
[0025] Preparation of 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutyronitrile hydrochloride (2)
[0026] Diphenylacetonitrile (5.8g, 30mmol) and 50% KOH aqueous solution (15ml), dimethylsulfoxide (DMSO) (100ml), tetrabutylammonium bromide (TBAB) (0.9g) and toluene 50ml were added to the reaction bottle, stirred at 40°...
example 2
[0032] Preparation of 1-(2-bromoethyl)-2-methyl-1H-imidazole (5)
[0033] 1,2-dibromoethane (50ml), 2-methylimidazole (2.5g, 30.5mmol), tetrabutylammonium chloride (0.43g) and Na 2 CO 3 (2.8g) and NaOH (3.3g) were sequentially added into a 100ml three-neck flask, stirred and heated to 40°C for 5h. After cooling to room temperature, the reaction solution was filtered, the filtrate was washed with saturated aqueous sodium bicarbonate solution, and dried over anhydrous sodium sulfate. Concentrate, add a mixed solvent of isopropyl ether and ethyl acetate (3:1) and stir to dissolve and crystallize to obtain 4.9 g of the product, with a yield of 85.1%, mp.79-80°C.
[0034] Preparation of 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutyronitrile hydrochloride (2)
[0035] Diphenylacetonitrile (5.8g, 30mmol) and 50% NaOH aqueous solution (15ml), dimethyl sulfoxide (DMSO) (100ml), tetrabutylammonium chloride (0.8g) and toluene 50ml were added to the reaction flask, Stir at 40 °C for 0.5 ...
example 3
[0041] Preparation of 1-(2-bromoethyl)-2-methyl-1H-imidazole (5)
[0042] 1,2-Dibromoethane (50ml), 2-methylimidazole (2.5g, 30.5mmol), benzyltriethylammonium chloride (TEBA) (0.35g) and Na 2 CO 3 (2.8g) and NaOH (3.3g) were sequentially added into a 100ml three-neck flask, stirred and heated to 45°C for 4h. After cooling to room temperature, the reaction solution was filtered, the filtrate was washed with saturated aqueous sodium bicarbonate solution, and dried over anhydrous sodium sulfate. Concentrate, add isopropyl ether and ethyl acetate mixed solvent (3:1) and stir to dissolve and crystallize to obtain 5.0 g of product, yield 86.8%, mp.79-80°C.
[0043] Preparation of 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutyronitrile hydrochloride (2)
[0044] Diphenylacetonitrile (5.8g, 30mmol) and 50% KOH aqueous solution (15ml), dimethylsulfoxide (DMSO) (100ml), benzyltriethylammonium chloride (TEBA) (0.66g) and toluene 50ml were added To the reaction flask, stirred at 40 ° C fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com