Method for synthesis of chlorothalonil artificial antigen
A technology of artificial antigen and synthesis method, which is applied in the direction of chemical instruments and methods, hybrid peptides, ovalbumin, etc., can solve the problems of cumbersome operation, inability to realize rapid detection of a large number of samples, long drug effect period, etc., and achieve simple synthesis steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
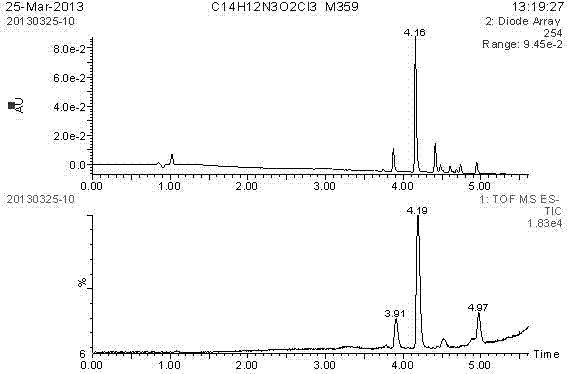
[0032] (1) Synthesis of hapten: Weigh 500mg (1.9mmol) of chlorothalonil, 106.6mg (1.9mmol) of KOH, and 262mg (2mmol) of 6-aminocaproic acid, dissolve these three drugs in 20mL of ethanol, 60℃ React for 12h, then react at 90°C for 12h. Rotate the reactant, then dissolve it with 20mL of water, adjust the pH to 4-5 with hydrochloric acid, produce a precipitate, and dry the precipitate to obtain the hapten CTNH. Identification and analysis were carried out by LC / MS.
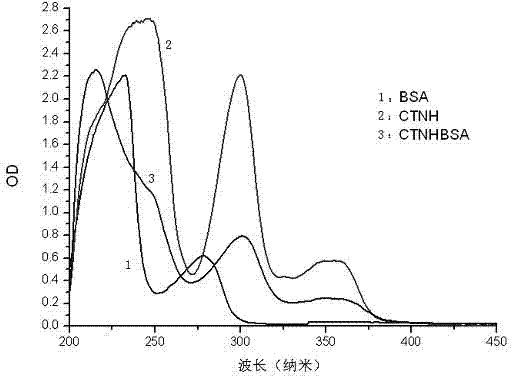
[0033] (2) Preparation of conjugate CTNH-BSA
[0034] Weigh 35.8 mg of CTNH, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) 56.9 mg, N-hydroxysuccinimide (NHS) 35.6 mg, and use 1 mL Anhydrous dimethylformamide (DMF) was dissolved (referred to as solution A), and stirred at room temperature for 8 hours. Weigh 112 mg of bovine serum albumin (BSA) and dissolve it in 20 mL of 0.01 mol / L PBS with pH 7.2 (referred to as solution B). Add solution A to solution B drop by drop at room temperature, and react overnight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com