Capecitabine pharmaceutical composition, and preparation method thereof
The technology of capecitabine and the composition is applied in the field of capecitabine pharmaceutical composition and preparation thereof, which can solve the problems of capecitabine being unstable when exposed to humidity, and achieve improved drug stability, fewer procedures, and simplified preparation. The effect of craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
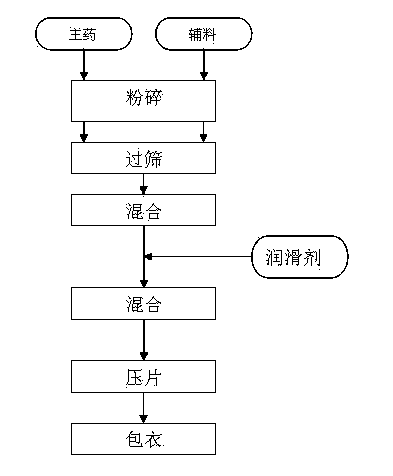
[0035] Preparation:
[0036] (1). Capecitabine 500mg, anhydrous lactose 10mg, microcrystalline cellulose 50mg, croscarmellose sodium 30mg, pulverize and sieve, mix well.
[0037] (2). 20 mg of micro-powdered silica gel is pulverized, sieved, and then fully mixed with the raw and auxiliary materials in step (1) and the powder is directly compressed into tablets.
[0038] (3). Take 25 mg of the film-coating composition, prepare a coating solution, and fluidize the plain tablet in (2), to obtain the final capecitabine film-coated tablet.
Embodiment 2
[0040] Preparation:
[0041] (1). Capecitabine 300mg, anhydrous lactose 5mg, microcrystalline cellulose 20mg, croscarmellose sodium 18mg, pulverize and sieve, mix well.
[0042] (2). 15 mg of micro-powdered silica gel is pulverized, sieved, and then fully mixed with the raw and auxiliary materials in step (1) and the powder is directly compressed into tablets.
[0043] (3). Take 18 mg of the film coating composition and prepare it into a coating solution, and fluidize the plain tablet in (2).
[0044] Obtain final capecitabine film-coated tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com