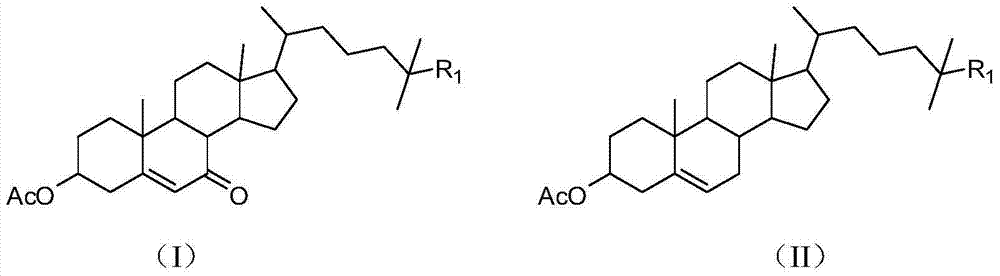
Preparation method of 7-ketone sterides
A compound and ketosteroid technology, which is applied in the field of preparing 7-keto steroids, can solve problems such as serious environmental pollution, and achieve the effects of less dosage, easy availability and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, a kind of preparation method of 7-ketocholesterol, carries out following steps successively:
[0039] Add 4.28g (0.01mol) of 3-acetylcholesterol and 1.63g (0.01mol) of NHPI into a three-necked flask, and at the same time add 60mL of acetone. After the 3-acetylcholesterol and NHPI are completely dissolved, add 5.14g ( 0.04mol) of tert-butyl hydroperoxide, while starting to stir, react at 20-25°C for 24h.
[0040] After the reaction, the reaction solution was distilled under reduced pressure (0.09MPa) to a yellow oil, 30mL of n-hexane was added and stirred again, heated to 40°C and stirred for 30 minutes, then cooled to room temperature, filtered, and the filter cake was the catalyst NHPI.
[0041] Distill the filtrate under reduced pressure (0.09MPa) until there is almost no n-hexane, then add 20mL of pyridine and 5mL of acetic anhydride, leave it overnight at room temperature, then distill under reduced pressure (0.09MPa), then add 10mL of methanol, and fi...
Embodiment 2-10
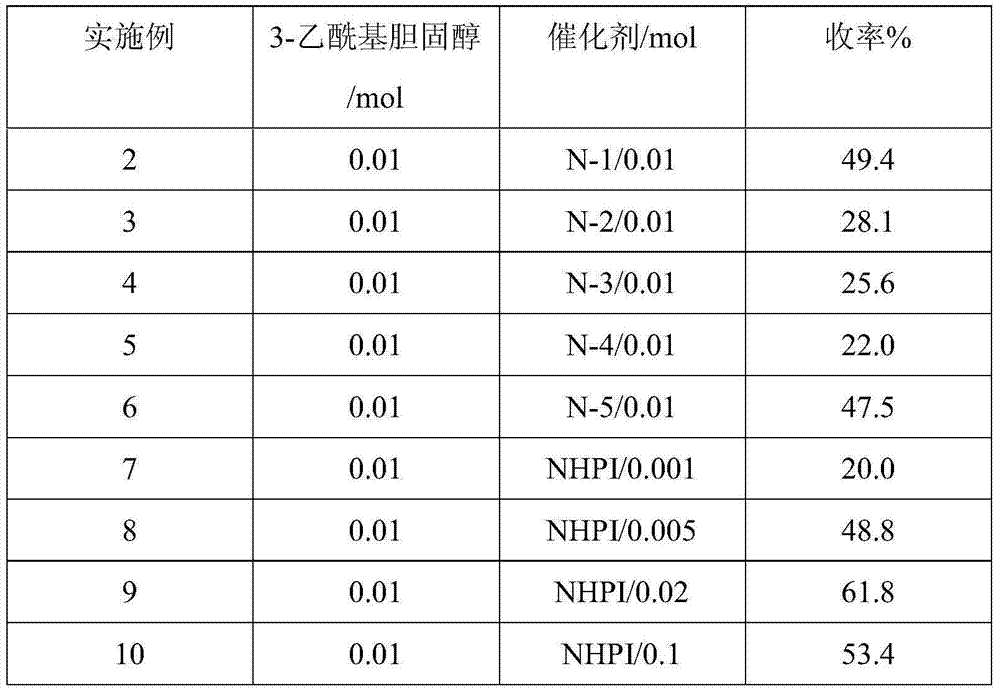
[0044] Change the kind and consumption of catalyst (as shown in table 1), all the other are equal to embodiment 1, obtain embodiment 2~10; The yield of gained product is as shown in table 1 below:
[0045] Table 1
[0046]
Embodiment 11-14
[0048] Change oxidizing agent---consumption and the reaction time of tert-butyl hydroperoxide, all the other are equal to embodiment 1, get embodiment 11~14; The yield of gained product is as shown in table 2 below:
[0049] Table 2
[0050] Example
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com