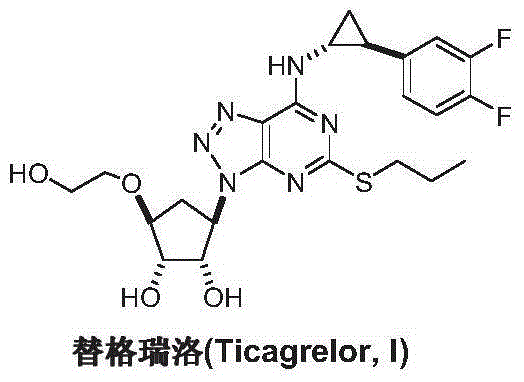
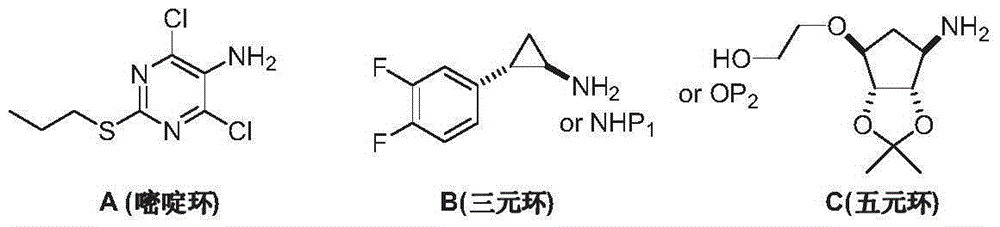
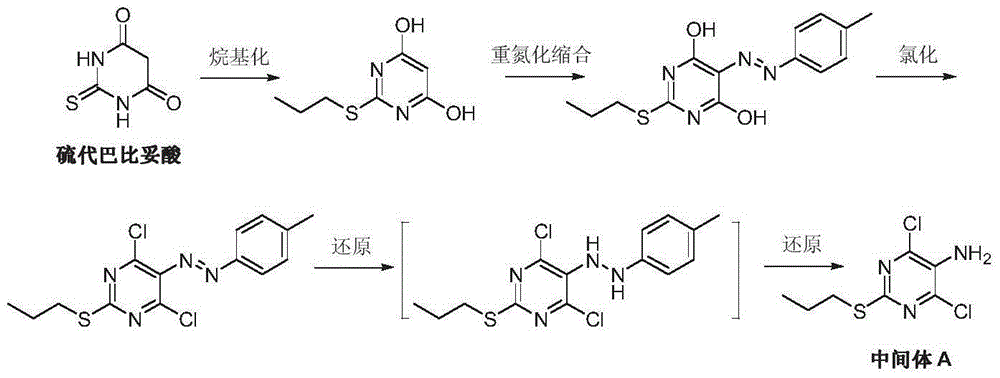
Preparation method of ticagrelor intermediate 4,6-dichloro-2-(propylmercapto)-5-aminopyrimidine
A technology of ticagrelor and aminopyrimidine, which is applied in the field of preparation of ticagrelor intermediate 4,6-dichloro-2--5-aminopyrimidine, can solve the problem of unfavorable industrialized production, great environmental protection and safety pressure and other problems, to achieve the effect of promoting development, promoting economy and technology, and simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 2-acetylamino-1,3-diethyl malonate (I) (10.9g, 50mmol), thiourea (4.76g, 62.5mmol, 1.25eq) and 100mL of methanol into a dry reaction flask, start stirring and Warm to reflux. 12 g of 30% methanol solution of sodium methoxide was added dropwise, and the reaction was kept under reflux for about 3 hours. TLC detected that the reaction was complete. Cool to room temperature, filter, wash the filter cake with cold methanol, and dry under vacuum at 50-55°C to obtain 10.5 g of off-white solid 5-acetylamino-2-thio-barbiturate sodium (III), yield 94.2%.
Embodiment 2
[0033] Add 2-benzyloxycarboxamido-1,3-diethyl-1,3-malonate (I) (15.5g, 50mmol), thiourea (4.76g, 62.5mmol, 1.25eq) and 150mL of methanol into a dry reaction flask, Stirring was started and the temperature was raised to reflux. 12 g of 30% methanol solution of sodium methoxide was added dropwise, and the reaction was kept under reflux for about 4 hours, and the reaction was detected by TLC. Cool to room temperature, filter, wash the filter cake with cold methanol, and dry under vacuum at 50-55°C to obtain 14.6 g of white solid 5-benzyloxycarboxamido-2-thio-barbiturate sodium (III), yield 92.7 %.
Embodiment 3
[0035] 5-Acetamido-2-thio-barbiturate sodium (III) (8.92g, 40mmol) was added to 50mL water and 50mL methanol solution in a reaction flask, and bromo-n-propane (IV) was added dropwise at room temperature (5.37g, 44mmol, 1.1eq), after stirring for 15 minutes, slowly add 20mL of 10% sodium hydroxide solution dropwise and keep at room temperature, continue to stir and react for 20 hours, and TLC detects that the reaction is complete. Add 100 mL of water, adjust the pH to 1.8-2.2 with dilute hydrochloric acid, and slowly stir for crystallization. After filtering, the filter cake was washed three times with water, and dried under vacuum at 55-60° C. to obtain 7.29 g of off-white solid 4,6-dihydroxy-5-acetylamino-2-(propylthio)pyrimidine (V), with a yield of 75.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com