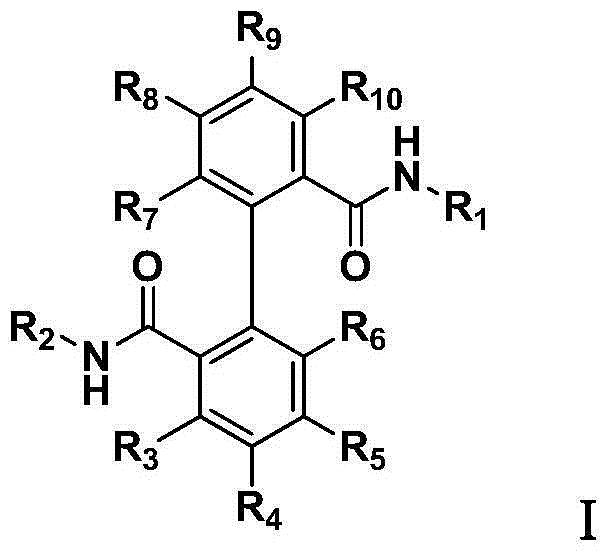
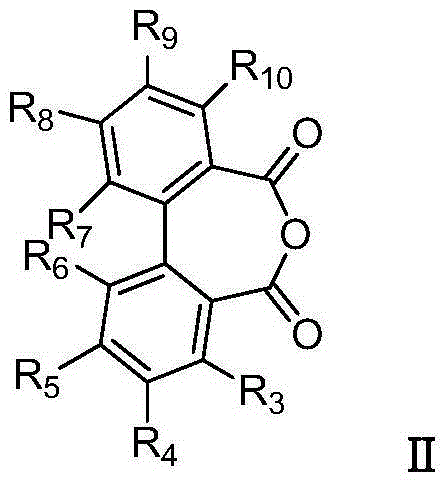
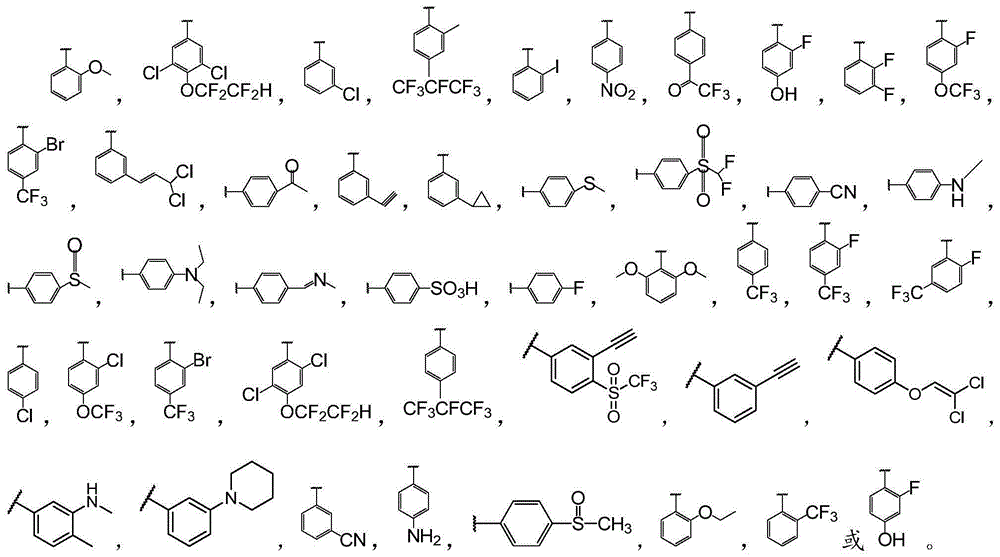
Biphenyl bisamide compounds and their preparation and use
A biphenyl bisamide and compound technology, which is applied in the field of biphenyl bisamide compounds, can solve the problems of serious pest resistance and few targets, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] (1) Preparation of 2'-formylmethylamino-[1,1'-biphenyl]-2-benzoic acid:
[0067]
[0068] Weigh 672 mg (3 mmol, 1 equivalent) of 2,2'-biphenyl dicarboxylic anhydride and put it in a round bottom flask, add 3 mL of DMF and 439 mg (3.6 mmol, 1.2 equivalent) of 2,4,6-collidine (catalyst), after stirring for 2 minutes, add 0.33ml of methylamine solution (3.3mmol, 1.1 equivalent, concentration 31%), stir at room temperature for 3 hours, stop the reaction, spin off DMF under reduced pressure, and dissolve the resultant with ethyl acetate, Then transferred to a separatory funnel, washed with 2M HCl, a large amount of solid precipitated out, suction filtered and recrystallized to obtain a white solid with a yield of 81%.
[0069] 1 HNMR(400MHz,DMSO)δ12.70(s,1H),8.71(d,J=4.7Hz,1H),7.59–7.46(m,3H),7.55–7.29(m,3H),7.15–7.05(m ,2H),2.63(d,J=4.6Hz,3H).
[0070] HRMS: m / z calculated value C 15 h 14 NO 3 (M+H) + 256.0968, measured value 256.0967
[0071] (2) Preparation of N-...
Embodiment 2
[0078] 4-Ethylthio-N-(2-methoxyphenyl)-N'-methyl-[1,1'-biphenyl]-2,2'-dicarboxamide (compound shown in formula Ⅰ-2 ) preparation:
[0079]
[0080] Divide by 4-ethylthio-2,2'-biphenyl dicarboxylic anhydride instead of 2,2'-biphenyl dicarboxylic anhydride (in step (1)) and 2-methoxyaniline instead of aniline (in step (2) Middle), the remaining steps were similar to Example 1 to obtain the title compound.
[0081] 1 HNMR(400MHz,DMSO)δ9.70(s,1H),8.58(d,J=4.6Hz,1H),7.87(dd,J=8.0,1.4Hz,1H),7.67–7.56(m,2H), 7.50–7.37(m,3H),7.13–7.08(m,1H),7.07–7.01(m,1H),7.01–6.94(m,1H),6.89–6.77(m,2H),3.56(s,3H ), 2.64(d, J=4.6Hz, 3H). 2.65(q, J=7.9Hz, 2H) 1.10(t, J=7.9Hz, 3H).
[0082] 13 CNMR(101MHz,DMSO)δ169.82,167.64,149.96,139.73,139.52,136.86,136.30,130.00,129.95,129.91,129.07,128.40,127.99,127.85,127.72,127.67,124.81,121.59,120.44,111.40,55.72,29.4, 26.47,14.1
[0083] HRMS: m / z calculated value C 24 h 25 N 2 o 3 S(M+H) + 421.1580, measured value 421.1582.
Embodiment 3
[0085] N-(3,5-dichloro-4-(1,1,2,2-tetrafluoroethoxy)phenyl)-N'-methyl-[1,1'-biphenyl]-2,2 Preparation of '-diformamide (compound shown in formula Ⅰ-3):
[0086]
[0087] Except that 3,5-dichloro-4-(1,1,2,2-tetrafluoroethoxy)aniline was used instead of aniline (in step (2)), the remaining steps were similar to Example 1 to obtain the title compound.
[0088] 1 HNMR(400MHz,DMSO)δ11.20(s,1H),8.62(d,J=4.7Hz,1H),7.67–7.59(m,3H),7.59–7.47(m,3H),7.47–7.39(m ,2H),7.23–7.08(m,2H),6.91(t,J=51.4Hz,1H),2.62(d,J=4.6Hz,3H).
[0089] 13 CNMR(101MHz,DMSO)δ170.86,168.24,139.40,139.31,138.96,136.21,136.11,136.09,130.63,130.12,130.05,130.00,129.79,128.25,128.23,128.18,127.51,119.58,26.54,22.52,14.41.
[0090] HRMS: m / z calculated value C 23 h 17 C l2 f 4 N 2 o 3 (M+H) + 515.0552 Found 515.0554.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com