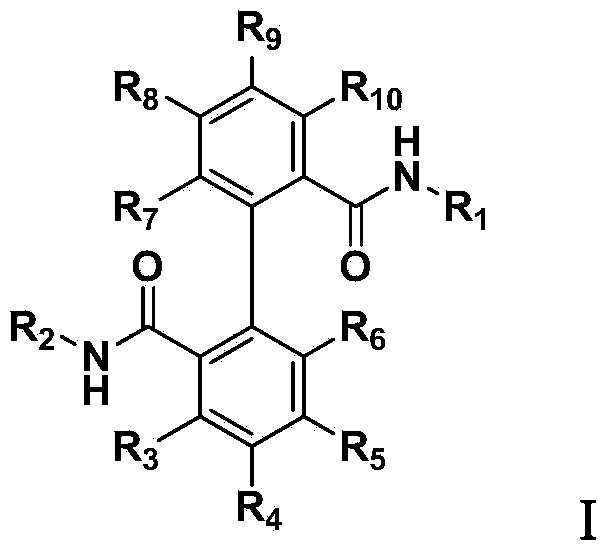
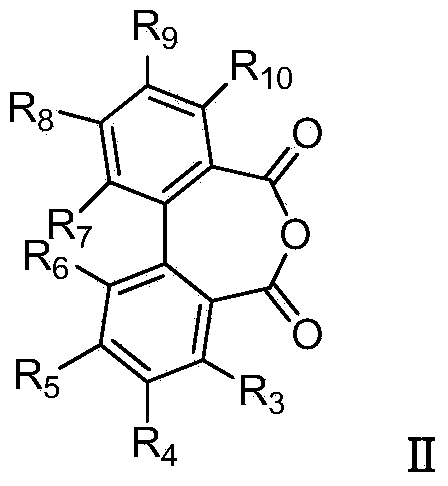
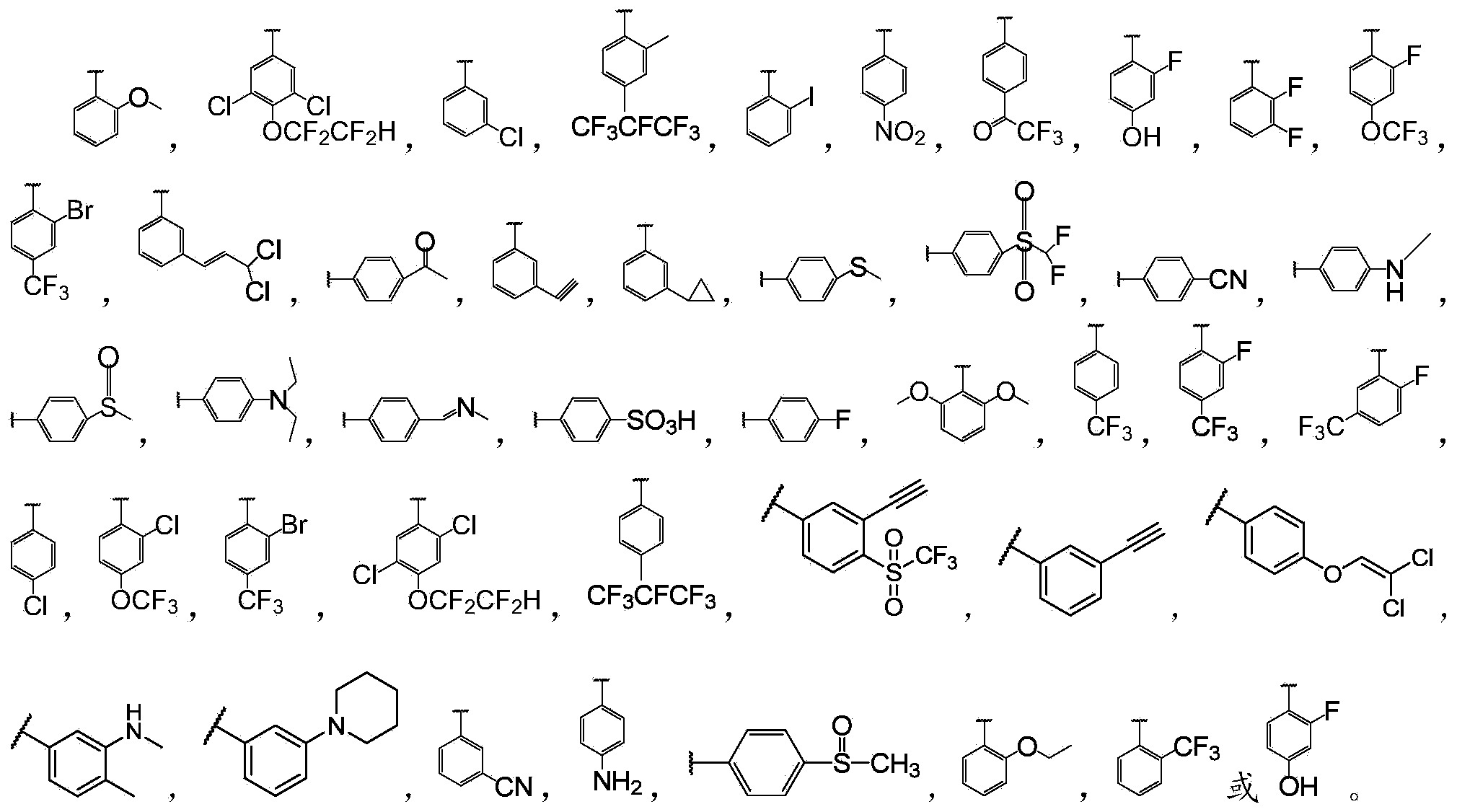
Biphenyl bisamide compound as well as preparation method and application thereof
A biphenyl bisamide compound technology, applied in the field of biphenyl bisamide compounds, can solve the problems of few targets and serious pest resistance problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] (1) Preparation of 2'-formylmethylamino-[1,1'-biphenyl]-2-benzoic acid:
[0067]
[0068] Weigh 672 mg (3 mmol, 1 equivalent) of 2,2'-diphthalic anhydride into a round bottom flask, add 3 mL of DMF and 439 mg (3.6 mmol, 1.2 equivalent) of 2,4,6-trimethylpyridine (Catalyst), after stirring for 2 minutes, add 0.33ml of methylamine solution (3.3mmol, 1.1 equivalent, concentration 31%), after stirring for 3 hours at room temperature, stop the reaction, spin off DMF under reduced pressure, and dissolve the resultant with ethyl acetate. Then it was transferred to a separatory funnel, washed with 2M HCl, and a large amount of solid was precipitated. The white solid was obtained by suction filtration and recrystallization. The yield was 81%.
[0069] 1 H NMR(400MHz,DMSO)δ12.70(s,1H), 8.71(d,J=4.7Hz,1H), 7.59-7.46(m,3H), 7.55-7.29(m,3H), 7.15-7.05( m,2H), 2.63(d,J=4.6Hz,3H).
[0070] HRMS: m / z calculated value C 15 H 14 NO 3 (M+H) + 256.0968, measured value 256.0967
[0071] (2) Prepa...
Embodiment 2
[0078] 4-Ethylthio-N-(2-methoxyphenyl)-N'-methyl-[1,1'-biphenyl]-2,2'-dimethylamide (the compound represented by formula I-2 ) Preparation:
[0079]
[0080] Divide by 4-ethylthio-2,2'-diphthalic anhydride instead of 2,2'-diphthalic anhydride (in step (1)) and 2-methoxyaniline instead of aniline (step (2) Except for middle), the remaining steps are similar to those in Example 1 to obtain the title compound.
[0081] 1 H NMR(400MHz,DMSO)δ9.70(s,1H), 8.58(d,J=4.6Hz,1H), 7.87(dd,J=8.0,1.4Hz,1H), 7.67-7.56(m,2H) ,7.50–7.37(m,3H),7.13–7.08(m,1H), 7.07–7.01(m,1H), 7.01–6.94(m,1H), 6.89–6.77(m,2H), 3.56(s, 3H), 2.64 (d, J=4.6 Hz, 3H) 2.65 (q, J=7.9 Hz, 2H) 1.10 (t, J=7.9 Hz, 3H).
[0082] 13 C NMR (101MHz, DMSO) δ169.82,167.64,149.96,139.73,139.52,136.86,136.30,130.00,129.95,129.91,129.07,128.40,127.99,127.85,127.72,127.67,124.81,121.59,120.44,111.40,55.72,29.4 ,26.47,14.1
[0083] HRMS: m / z calculated value C 24 H 25 N 2 O 3 S(M+H) + 421.1580, the measured value is 421.1582.
Embodiment 3
[0085] N-(3,5-Dichloro-4-(1,1,2,2-tetrafluoroethoxy)phenyl)-N'-methyl-[1,1'-biphenyl]-2,2 '-Diformamide (the compound represented by formula I-3):
[0086]
[0087] Except that 3,5-dichloro-4-(1,1,2,2-tetrafluoroethoxy)aniline was substituted for aniline (in step (2)), the remaining steps were similar to those in Example 1 to obtain the title compound.
[0088] 1 H NMR(400MHz,DMSO)δ11.20(s,1H), 8.62(d,J=4.7Hz,1H), 7.67-7.59(m,3H), 7.59-7.47(m,3H), 7.47-7.39( m, 2H), 7.23-7.08 (m, 2H), 6.91 (t, J=51.4Hz, 1H), 2.62 (d, J=4.6Hz, 3H).
[0089] 13 C NMR (101MHz, DMSO) δ 170.86,168.24,139.40,139.31,138.96,136.21,136.11,136.09,130.63,130.12,130.05,130.00,129.79,128.25,128.23,128.18,127.51,119.58,26.54,22.52,14.41.
[0090] HRMS: m / z calculated value C 23 H 17 C l2 F 4 N 2 O 3 (M+H) + 515.0552 The measured value is 515.0554.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com