Vasospasm preventing nanometer fiber membrane and preparing method thereof
A nanofibrous membrane and vasospasm technology, applied in the field of biomedicine, can solve problems such as difficulty in ensuring continuous drug dosage, affecting normal organs or tissues, frequent injection of blood vessels, etc., to achieve prevention and treatment of vasospasm, flexibility and stretchability Good, good biodegradability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
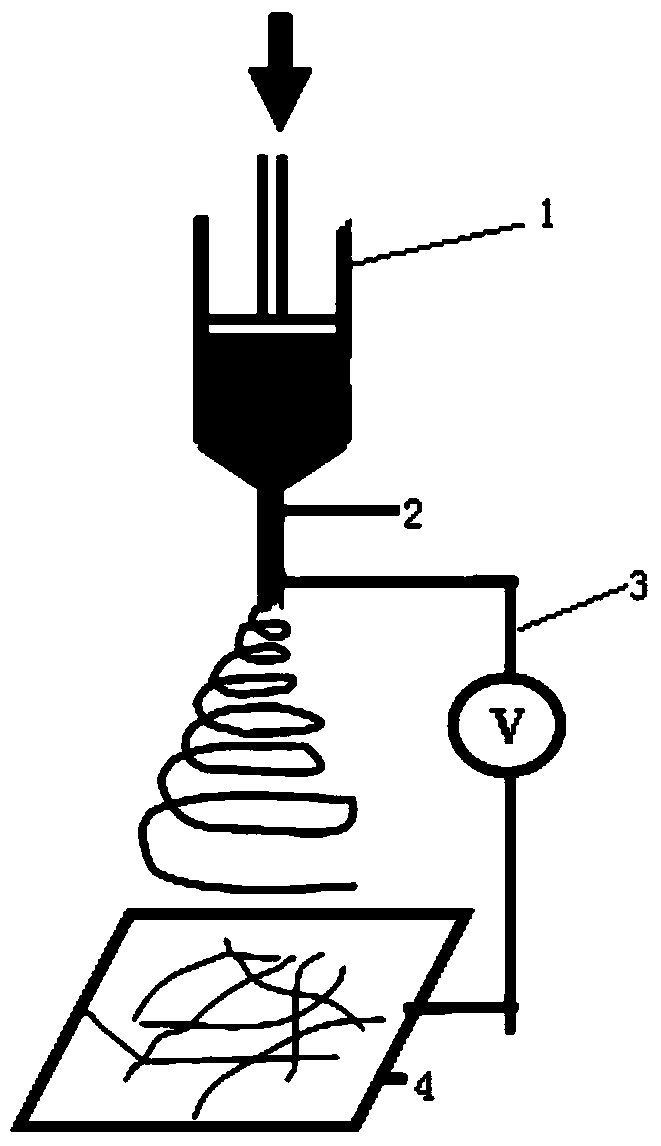
[0037] In the present invention, the preparation method of the degradable membrane carrier preferably includes:
[0038] The degradable material is dissolved in an organic solvent to prepare a degradable material solution, and then electrospun to prepare the degradable membrane carrier.
[0039]In the present invention, the types of the degradable material and the organic solvent are consistent with the types of the degradable material and the organic solvent described in the above technical solution, and will not be repeated here; the mass ratio of the degradable material to the organic solvent is preferably 1:2-20, more preferably 1:1:4-15, most preferably 1:6-10. In the present invention, the temperature of the electrospinning is preferably 0°C to 35°C, more preferably 15°C to 30°C, most preferably 20°C to 25°C; the flow rate of the electrospinning is preferably 0.3mL / h~0.8mL / h, more preferably 0.4mL / h~0.7mL / h, most preferably 0.5mL / h~0.6mL / h; the voltage of the electrosp...
Embodiment 1
[0070] 0g, 0.1111g, 0.25g, 0.4285g, 0.6666g and 1g of polyethylene glycol (PEG) were mixed with 1g of poly(L-lactic acid) (PLLA) respectively to obtain PLLA-PEG in different mass ratios;
[0071] Dissolving the above-mentioned PLLA-PEG in different mass ratios in a mixed solution of 2 g dimethylformamide and 4 g methylene chloride to obtain PLLA-PEG solutions in different mass ratios;
[0072] Dissolve 0.06g papaverine hydrochloride in 0.5mL hexafluoroisopropanol and then dissolve it in the above-mentioned PLLA-PEG solutions with different mass ratios to obtain the electrospinning solution, which are named PLLA and PLLA respectively according to the mass ratios of PLLA and PEG. -10%, PLLA-20%, PLLA-30%, PLLA-40% and PLLA-50%;
[0073] Set the voltage in the electrospinning technical parameters to 10KV, the flow rate to 0.5mL / min, the distance from the needle to the collecting plate to 15cm, the temperature to 25°C, and the relative humidity to 50%. The above obtained PLLA, PLL...
Embodiment 2
[0108]0g, 0.1111g, 0.25g, 0.4285g, 0.6666g and 1g of polyethylene glycol (PEG) were mixed with 1g of poly(L-lactic acid) (PLLA) respectively to obtain PLLA-PEG in different mass ratios;
[0109] Dissolving the above-mentioned PLLA-PEG with different mass ratios in a mixed solution of 2g tetrahydrofuran and 4g chloroform to obtain PLLA-PEG solutions with different mass ratios;
[0110] Dissolve 0.9g of papaverine hydrochloride in 0.5mL of ethanol and then dissolve it in the PLLA-PEG solutions with different mass ratios above to obtain the electrospinning solution, which are respectively named PLLA, PLLA-10%, PLLA-20%, PLLA-30%, PLLA-40% and PLLA-50%;
[0111] Set the voltage in the electrospinning technical parameters to 50KV, the flow rate to 0.5mL / min, the distance from the needle to the collecting plate to 15cm, the temperature to 35°C, and the relative humidity to 50%. The above obtained PLLA, PLLA-10%, PLLA- 20%, PLLA-30%, PLLA-40% and PLLA-50% electrospinning solutions w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Fiber diameter | aaaaa | aaaaa |
| Strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com