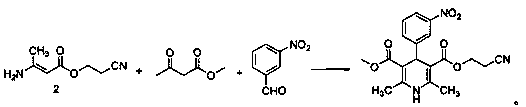
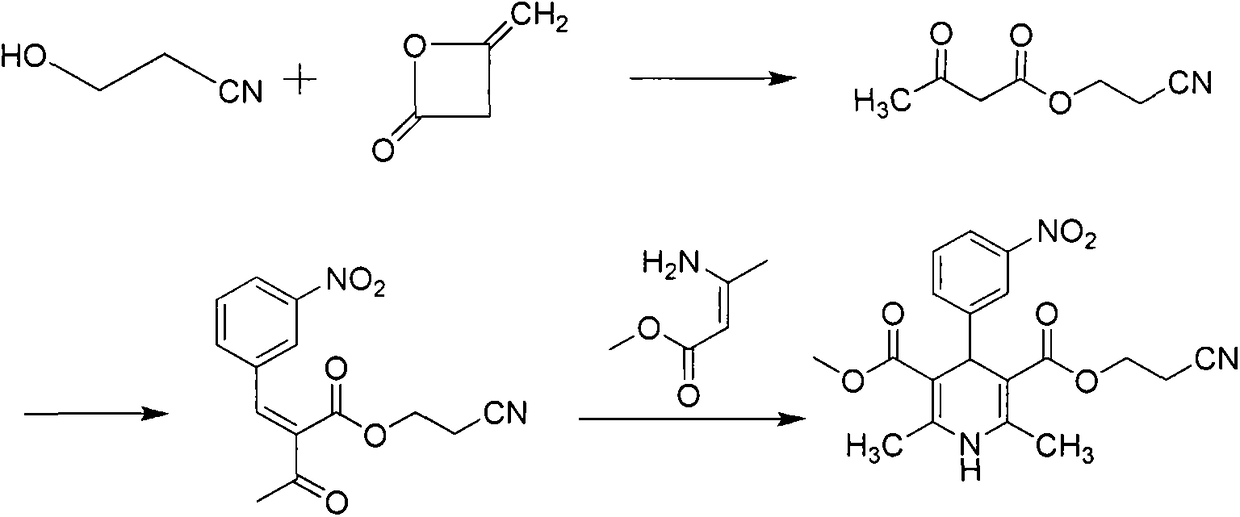
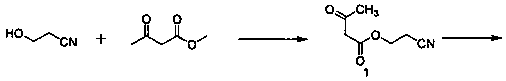3-(2-Nitroethyl)-5-methyl-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate preparation method
A technology of nitrophenyl and dihydropyridine, applied in the field of pharmacy, can solve the problems of unsuitable industrial production, low yield, complicated preparation method, etc., and achieve the effects of shortening production cycle, good appearance and simplifying operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, the synthesis of cyanoethyl acetoacetate:
[0029] 28.4g of 3-hydroxypropionitrile, 46.4g of methyl acetoacetate, and 14.2g of N-bromosuccinimide (NBS) were added to a 250ml reaction flask, 150ml of toluene was added, and the temperature was raised to 90°C. After reacting for 3 hours, it was cooled to room temperature, filtered, and the filtrate was washed three times with water and then spin-dried to obtain 59 g of a yellow liquid with a yield of 95.2% and a purity of 92% by HPLC.
Embodiment 2
[0030] Embodiment 2, the synthesis of cyanoethyl acetoacetate
[0031] 28.4g of 3-hydroxypropionitrile, 46.4g of methyl acetoacetate, and 7.1g of N-bromosuccinimide (NBS) were added to a 250ml reaction flask, 150ml of toluene was added, and the temperature was raised to 90°C. After reacting for 3 hours, it was cooled to room temperature, filtered, and the filtrate was washed three times with water and then spin-dried to obtain 58.6 g of a yellow liquid with a yield of 94.6% and an HPLC purity of 86%.
Embodiment 3
[0032] Embodiment 3, the synthesis of cyanoethyl acetoacetate
[0033] Add 28.4g of 3-hydroxypropionitrile, 46.4g of methyl acetoacetate, and 10g of N-bromosuccinimide (NBS) into a 250ml reaction flask, add 150ml of toluene, heat up to 90°C, and react After 3 hours, it was cooled to room temperature, filtered, and the filtrate was washed three times with water and then spin-dried to obtain 59.5 g of a yellow liquid with a yield of 95.9% and an HPLC purity of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com