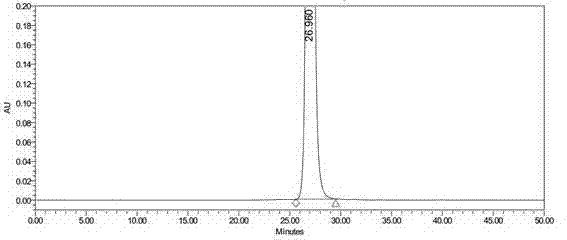
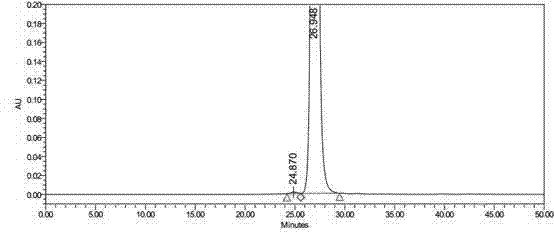
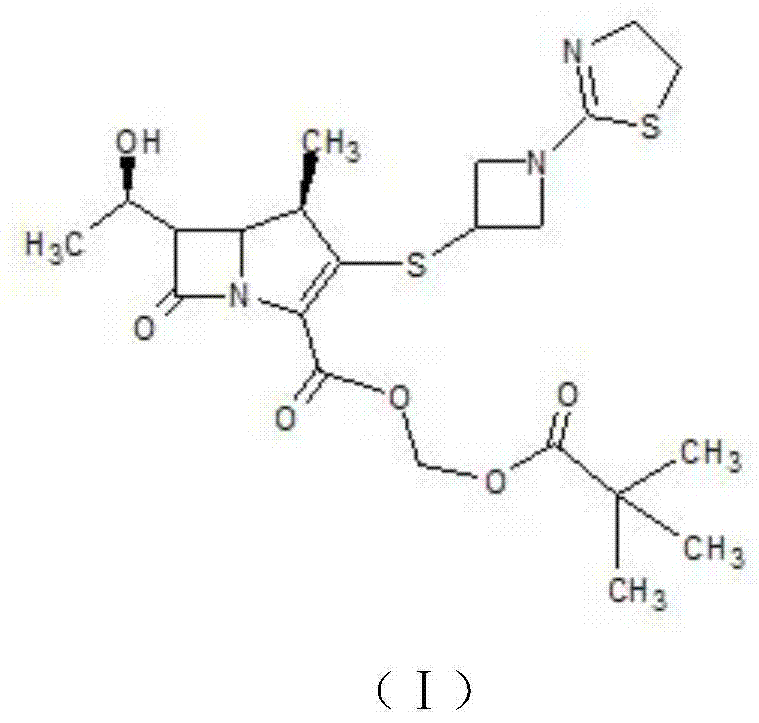
Synthetic method for Tebipenem Pivoxil polymer impurity
A technology of tibipenem ester and synthesis method, which is applied in the field of preparation of polymer impurities, and can solve problems such as difficulty in purification, complex product components, and inability to be used in qualitative and quantitative research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 T24
[0025] Add 200ml DMF, tibipenem (20g, 78.1mmol), triethylamine (17.34g, 156.2mmol), DMAP (0.3g, 2.6mmol), to the reaction flask in turn, cool down to 0°C, add TBSCl (45.2mmol) in batches g, 299.9 mmol). Complete the heat preservation at 0°C for 2 to 3 hours. Add EA / H to the reaction vial 2 O300ml / 300ml, liquid separation, the aqueous phase was extracted twice with ethyl acetate 300ml, the organic phase was combined, washed twice with saturated brine 300ml, the organic phase was dried with anhydrous magnesium sulfate, concentrated, purified by silica gel chromatography, and successively used Petroleum ether, ethyl acetate / petroleum ether (1 / 20), and ethyl acetate were eluted, and the eluate of the target compound was collected and concentrated to obtain the product (31.0g, 62.48mmol).
Embodiment 2
[0026] The preparation of embodiment 2 P10
[0027] Add 120ml DMF, P9 (20g, 49.8mmol), triethylamine (16ml, 115mmol), triethylbenzyl ammonium chloride (20g, 71.9mmol) to the reaction flask, heat up to 40-50°C, and add pivalic acid dropwise Chloromethyl ester (16ml, 106.6mmol), react for 1~2h. Add ethyl acetate / water 120ml / 120ml to the reaction bottle, separate the liquids, extract the aqueous phase with 120ml ethyl acetate, combine the organic phases, wash twice with saturated brine 120ml, dry the organic phases with anhydrous magnesium sulfate, and then concentrate. Purified by silica gel chromatography, eluted with ethyl acetate / petroleum ether (1 / 10), ethyl acetate / petroleum ether (1 / 5), ethyl acetate / petroleum ether (1 / 3) in sequence, and collected the fraction of the target compound The eluate was concentrated to give the product (25 g, 39.7 mmol).
Embodiment 3
[0028] The preparation of embodiment 3 T26
[0029] In a 250ml three-necked flask, add T24 (497mg, 1mmol), HOBt (152mg, 1mmol) and 50ml of DMF, cool down to -10°C, add DCC (206mg, 1mmol), stir for 30 minutes, add P10 (567mg, 0.9mmol) 10ml of DMF solution, complete the reaction at -10-0°C for 2-3 hours. Add 100ml of water / 100ml of ethyl acetate to the reaction solution for extraction, wash the organic phase with 50ml of water three times, dry the organic phase with anhydrous magnesium sulfate, and concentrate to dryness under reduced pressure. Use silica gel column chromatography, gradient elution with ethyl acetate / petroleum ether (1 / 20~1 / 1), collect the eluate of the target compound and concentrate to obtain T26 (942mg, 0.85mmol).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com