Targeted thymidine kinase photosensitizer, pharmaceutical composition thereof, and applications of targeted thymidine kinase photosensitizer in treating cancers
A technology of thymidine kinase and photosensitizer, which is applied in the field of targeted thymidine kinase photosensitizer and its pharmaceutical composition, can solve the problems of large limitations, single prostate cancer, and unsatisfactory effect, and achieve enhanced selectivity and tumor expansion Range of application, well tolerated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of HPPH-thymidine conjugates
[0057]
[0058] Wherein, the number of HPPH is compound 1, and the number of HPPH-thymidine conjugate is compound 3; the specific preparation method is as follows:
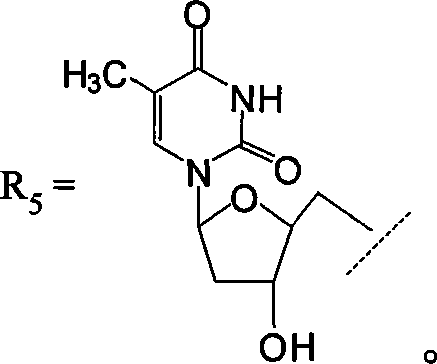
[0059] HPPH (300mg, 0.48mmol), benzotriazol-1-yl-oxytri(dimethylamino)phosphonium hexafluorophosphate (255mg, 0.58mmol), thymidine (1800mg, 7.2mmol) and Triethylamine (about 0.5 mL) was dissolved in about 20 mL of anhydrous dimethylformamide (DMF), and the reaction mixture was stirred overnight. After removal of DMF under high vacuum, the 2 Cl 2 This mixture is used as eluent to purify, and the target product yield obtained reaches more than 52% (210mg), and its electronic absorption spectrum is as attached figure 1 As shown (UV-Vis, methanol concentration is 7.7 μM), λ max (MeOH), nm (ε): 663nm (5.25×10 4 ), 606nm (7.49×10 3 ), 538nm (7.51×10 3 ), 507nm (7.28×10 3 ), 412nm (10.52×10 4 ).
[0060] NMR attached figure 2 as shown, 1 HNMR (CD...
Embodiment 2
[0061] Example 2: Preparation of targeted thymidine kinase photosensitizer containing substituted phenyl
[0062] Targeted thymidine kinase photosensitizer containing substituted phenyl group ie 17 3 -Thymidine-20-(4-thymidyloxycarbonyl)phenyl-3-((1'-n-hexyloxy)ethyl)-3-desvinyl-pyropheophorbide preparation route and Concrete experiment is as follows (the present invention numbers it as compound 6):
[0063]
[0064] Step 1, the preparation of compound 4:
[0065] HPPH1 (300 mg, 0.47 mmol) and pyridinium tribromide (196 mg, 0.61 mmol) were dissolved in 10 mL of dichloromethane; 3 drops of pyridine were added to the reaction mixture. The reaction mixture was stirred for 40 minutes. After work-up, chromatograph using 5% MeOH / CH 2 Cl 2 The mixture was purified as eluting solvent. The target compound was obtained in 48% yield (160 mg). UV-visible light, λ max (CH 2 Cl 2 ), nm(ε): 672nm (4.65×10 4 ), 552nm (1.69×10 4 ), 418nm (11.1×10 4 ). 1 HNMR (CDCl 3 ; 400MHz)...
Embodiment 3
[0074] Example 3: Preparation of Targeted Thymidine Kinase Photosensitizer Containing Additional N Heterocycles
[0075] Preparation line:
[0076]
[0077] details as follows:
[0078] 3-(1-(n-butoxy)ethyl)purpurin-18-N-butyramide-17-propionic acid 7 (120mg, 0.173mmol), BOP (169mg, 0.38mmol), thymidine (1050mg , 4.32 mmol) and triethylamine (about 0.5 mL) were dissolved in approximately 15 mL of anhydrous DMF, and the reaction mixture was stirred overnight; after removal of DMF under high vacuum, the 2 Cl 2 The mixture was purified as the eluting solvent to obtain the target compound in 51% yield (80 mg). UV-visible light, λ max (MeOH), nm(ε): 699nm (4.51×10 4 ), 642 (7.31×10 3 ), 543 (1.79×10 4 ), 507 (7.29×10 3 ), 413 (12.52×10 4 ). 1 HNMR (CDCl 3 ; 400MHz): δ9.79, (s, 1H, H-5), 9.66 (s, 1H, H-10), 8.53 (s, 1H, H-15), 7.21 (s, 1H, ArH), 7.03 (m, 1H, ), 6.80(m, 3H, ), 6.22(m, 3H, ), 5.80 (q, J=6.5Hz, 1H, 3 1 -H), 5.37(m, 2H, -NCH 2 (CH 2 ) 2 CH 3 )...
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com