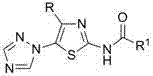
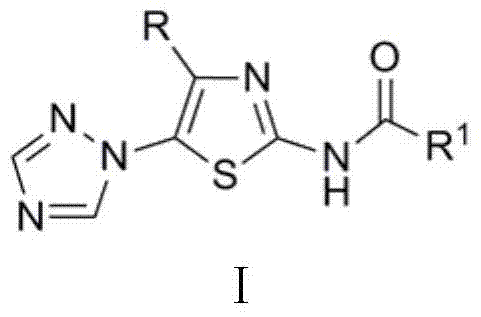
Medical application of N-{5-(1,2,4-triazole-1-yl) thiazole-2-yl} fatty acid amide
A kind of technology of fatty amide and thiazole, which is applied in the application field of preparing influenza virus neuraminidase inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0007] N - Preparation of [4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-yl]acetamide
[0008] 2 mmol 4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-amine, 6.0 mL acetic anhydride, reacted at 50°C for 1.5 h, cooled, poured into ice water and stirred to obtain N -[4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-yl]acetamide, yield 90.6%, m.p. 190~191℃. 1 H NMR (400 MHz, CDCl 3 ) δ : 1.13 (s, 9H, 3 x CH 3 ), 2.29 (s, 3H, CH 3 ), 8.12 (s, 1H, C 2 N 3 h 2 3-H), 8.28 (s, 1H, C 2 N 3 h 2 5-H), 9.16 (s, 1H, NH).
Embodiment 2
[0010] N Preparation of -[4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-yl]propionamide
[0011] According to the method of Example 1, 4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-amine reacted with propionic anhydride for 0.5 h to obtain N -[4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-yl]propionamide, yield 87.2%, m.p. 159~161℃. 1 H NMR (400 MHz, CDCl 3 ) δ : 1.13 (s, 9H, 3 x CH 3 ), 1.28(t, J = 7.6 Hz, 3H, CH 3 ), 2.54 (q, J = 7.6 Hz, 2H, CH 2 ), 8.15 (s, 1H, C 2 N 3 h 2 3-H), 8.31 (s, 1H, C 2 N 3 h 2 5-H), 9.49 (s, 1H, NH).
Embodiment 3
[0013] N - Preparation of [4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-yl]-2-butenamide
[0014] 2 mmol 4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-amine was dissolved in 20.0 mL dichloromethane, 2.2 mmol 2-butenoic acid, 0.03 g 4 - Dimethylaminopyridine (DMAP), add 2.2 mmol N, N'-dicyclohexylcarbodiimide (DCC) after 0.5 h, stir at room temperature, react for 6.0 h, the reaction solution is neutralized with aqueous sodium bicarbonate solution, and allowed to stand , layered, the organic layer was dried with anhydrous sodium sulfate, filtered, rotary evaporated, and column chromatography was obtained N -[4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-yl]-2-butenamide, yield 35.8%, m.p. 205~208℃. 1 H NMR (400 MHz, CDCl 3 ) δ : 1.15 (s, 9H, 3 x CH 3 ), 1.98 (dd, J = 7.0 Hz, J = 1.6 Hz, 3H, CH 3 ), 5.99 (dd, J = 14.2 Hz, J = 1.6 Hz, 1H, 3'-H), 7.13-7.19 (m, 1H, 2'-H), 8.12 (s, 1H, C 2 N 3 h 2 3-H), 8.26 (s, 1H, C 2 N 3 h 2 5-H), 8.92 (s, 1H, NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com