Method for preparing R-isomer by using S-isomer of budesonide
A technology of isomers and systems, applied in the field of preparation of highly active R isomers, can solve problems affecting product yield and achieve good recovery and high conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
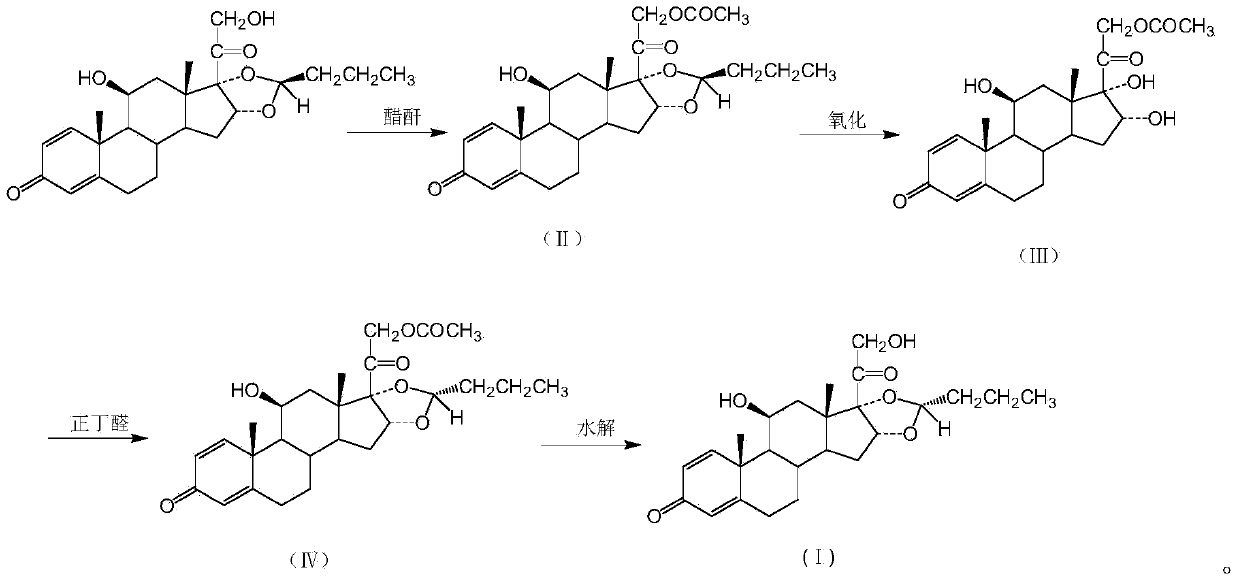
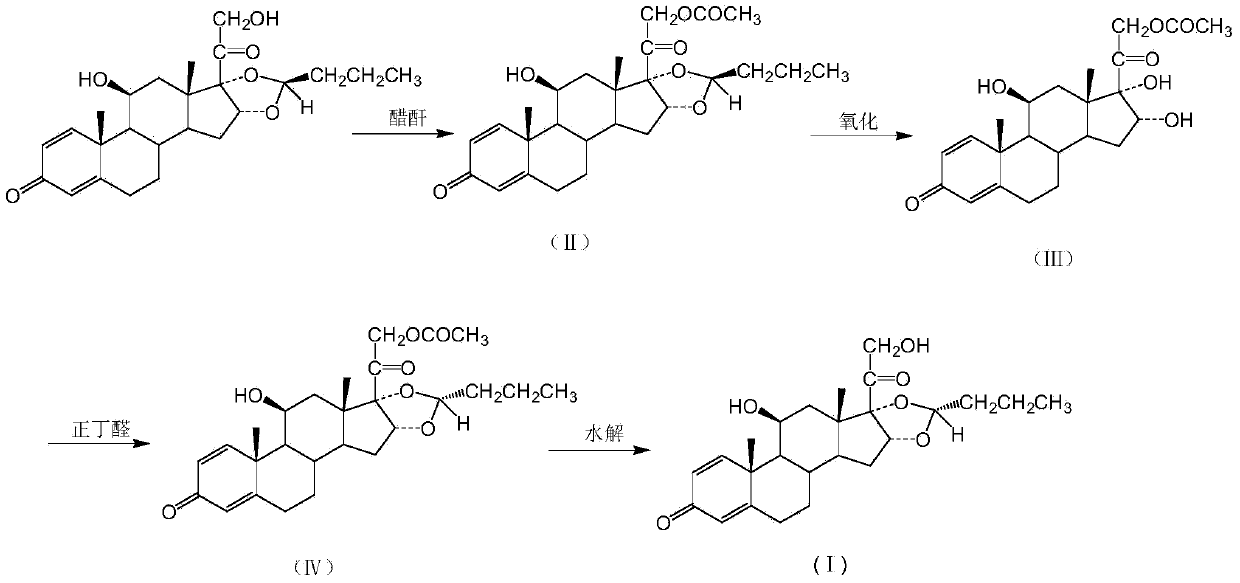
[0018] Put 50g of recovered budesonide (R / S=20 / 80) into the reaction kettle, add 200ml of dichloromethane, stir and dissolve completely. Then the temperature was lowered to 0°C, and 15 ml of acetic anhydride was added dropwise. After dropping, keep warm for 30 minutes. Then it was washed with aqueous sodium bicarbonate solution, and the layers were separated. The dichloromethane layer was concentrated to dryness under reduced pressure to obtain budesonide acetate, formula II, yield.
Embodiment 2
[0020] Put 57g of budesonide acetate obtained in Example 1 into the reaction kettle, add 900ml of methanol, 10ml of formic acid, and heat to dissolve completely. Then the temperature was lowered to 10°C, 220ml of 10% potassium permanganate aqueous solution was added dropwise, and the reaction was incubated for 1.5 hours after the drop was completed. After the reaction was complete, it was filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 16-α-hydroxyprednisolone acetate, formula III, with a yield of 85%.
[0021] At the same time, potassium dichromate and chromium trioxide were used instead of potassium permanganate, and the oxidation ring-opening reaction was carried out under the same conditions, and the yields were 76% and 81%, respectively.
Embodiment 3
[0023] Put 600ml of perchloric acid into the reaction kettle, ventilate with nitrogen, and stir. Cool down to -20°C and add 23ml of n-butyraldehyde, then add 48.7g of the budesonide intermediate obtained in Example 2 while maintaining the temperature. The reaction was incubated for 3 hours. After the reaction was complete, the reaction solution was poured into 5 L of water, filtered, and the filter cake was recrystallized with ethanol to obtain 32.4 g of dexbudesonide acetate with a yield of 64.8%.
[0024] R isomer: 99.2%
[0025] S isomer: 0.8%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com