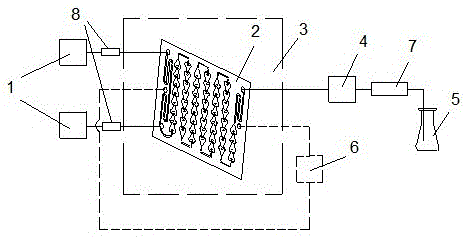
Method for synthesizing 2,2,4-trimethyl-1,2-dihydroquinoline and polymer thereof using microchannel reactor
A technology of microchannel reactor and dihydroquinoline, which is applied in the direction of organic chemistry, can solve the problems of severe corrosion, long reaction time, and large volume, and achieve the effects of low risk, short reaction time, and easy industrial scale-up production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Take 1 mol of hydrochloric acid, 6 mol of aniline, and 24 mol of acetone, first react aniline and hydrochloric acid to obtain aniline hydrochloride, and fully mix it with acetone. Turn on the heat carrier circulation control system, set the reaction temperature to 110°C, use the metering pump to inject 6 pieces of microchannel reactors in series at a flow rate of 30ml / min for reaction, and adjust the system pressure to 8~10bar through the backup pressure valve. The reaction solution stays in the microchannel reactor for 120s, and the resulting reaction solution is distilled under normal pressure to remove unreacted excess acetone. The reaction solution sample is taken and analyzed by high-performance liquid chromatography. The relative content of the monomer is 21.39%, and its polymer is relatively The content is 4.69%, and the relative content of aniline is 60.25%.
Embodiment 2
[0031] Get 1mol hydrochloric acid, 6mol aniline, 36mol acetone, reaction operation condition is the same as embodiment 1, analyze reaction solution through high performance liquid chromatography, wherein monomer relative content is 33.69%, its polymer relative content is 13.33%, aniline relative content is 36.57% %.
Embodiment 3
[0033] Get 1mol hydrochloric acid, 6mol aniline, 42mol acetone, reaction operation condition is the same as embodiment 1, analyze reaction solution through high performance liquid chromatography, wherein monomer relative content is 33.81%, its polymer relative content is 10.26%, aniline relative content is 40.13% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com