Trospium chloride slow-release composition and preparation method thereof
A slow-release composition, trospium chloride technology, applied in the direction of drug combination, heterocyclic compound active ingredients, drug delivery, etc., can solve the problems of talc powder irritation, etc., to achieve long-term treatment, small side effects, blood medicine Concentration stabilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
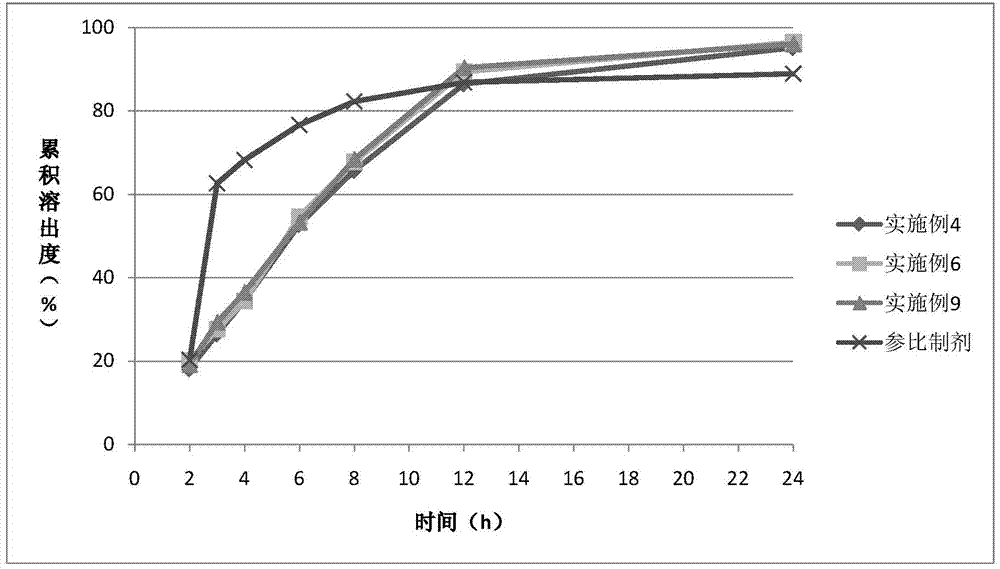
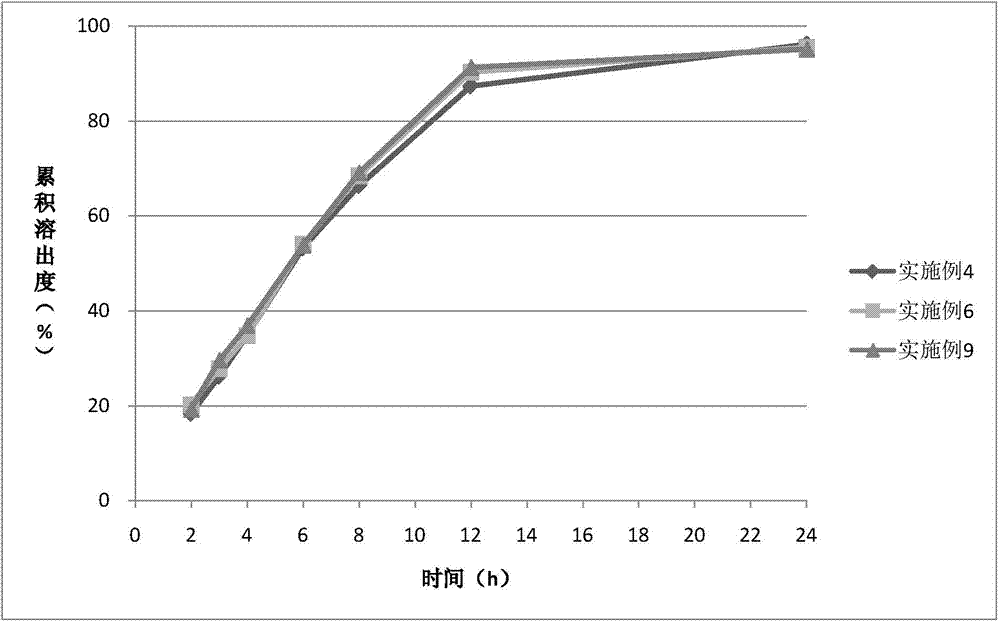
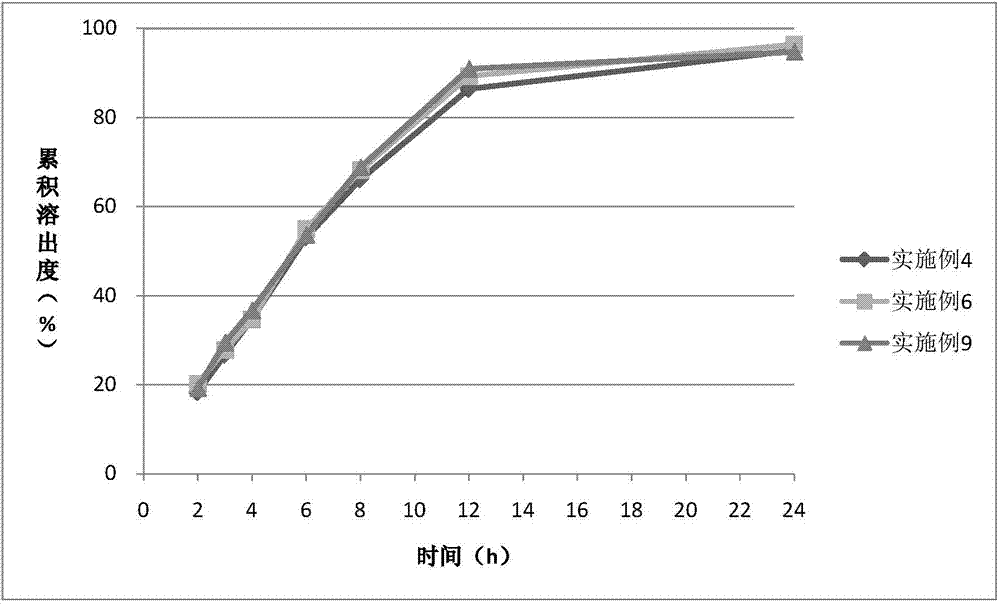
Examples
Embodiment 1
[0046] The trospium chloride sustained-release composition was prepared by the following preparation method.
[0047] (1) Pill core drug loading: Weigh trospium chloride raw material, adhesive and anti-adhesive agent according to the ratio shown in Table 1, and disperse them in an ethanol aqueous solution with a volume percentage of 80%, and prepare A coating solution with a weight volume percentage content of 20% is coated on the blank ball core shown in Table 1 by a fluidized bed (Chongqing Jinggong Pharmaceutical Machinery Co., Ltd., DPL1 / 3), and the coating solution is Coated on the blank pellet core to prepare drug-loaded pellets.
[0048] (2) Coating of the isolation layer: Weigh Opadry according to the ratio shown in Table 1, disperse it in an aqueous ethanol solution with a volume percentage of 80%, and make a suspension with a weight volume percentage of 5%. liquid, the drug-loaded pellets prepared in step (1) are coated by a fluidized bed process, and the suspension...
Embodiment 2
[0052] The trospium chloride sustained-release composition was prepared by the following preparation method.
[0053] (1) Pill core drug loading: Weigh trospium chloride raw material, adhesive and anti-adhesive agent according to the proportion shown in Table 1, disperse them in 50% ethanol aqueous solution by volume, and prepare the weight The coating solution with a volume percentage content of 40% is coated on the blank ball core shown in Table 1 by using a fluidized bed (Chongqing Jinggong Pharmaceutical Machinery Co., Ltd., DPL1 / 3) process, and the coating solution is coated Cover the blank ball core to prepare drug-loaded micropills.
[0054] (2) Coating of the isolation layer: Weigh Opadry according to the ratio shown in Table 1, disperse it in an aqueous ethanol solution with a volume percentage content of 50%, and make a suspension with a weight volume percentage content of 5%. liquid, the drug-loaded pellets prepared in step (1) are coated by a fluidized bed process...
Embodiment 3
[0058] According to the proportioning shown in Table 1, the trospium chloride sustained-release composition was prepared according to the method of Example 1, but in the step (1), the ethanol aqueous solution with a volume percentage content of 70% was used, and the weight of the coating solution configured was The volume percentage content is 25%. Then, the prepared trospium chloride sustained-release composition is added with conventional auxiliary materials, and compressed into trospium chloride sustained-release tablets with a specification of 50 mg using conventional techniques.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com