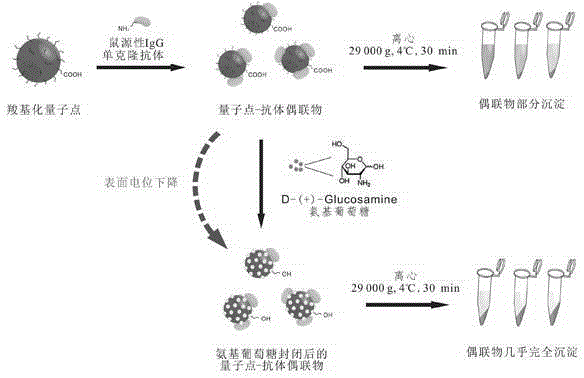
A method for efficient purification of quantum dots and igg-like monoclonal antibody conjugates
A monoclonal antibody and quantum dot technology, applied in the biological field, can solve problems such as harsh operating conditions, complicated process, and low yield, and achieve the effects of low equipment requirements, efficient separation, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 water-soluble quantum dot anti-aflatoxin B 1 Monoclonal antibody conjugates and purification process
[0032] Take 5mL of commercial carboxylated water-soluble quantum dots (concentration: 50nmol / L) and mix it with an equal volume of 0.05mol / L borate buffer at pH 6.0; Base-(3-dimethylaminopropyl) carbodiimide and N-hydroxysulfosuccinimide were reacted at 37°C for 2 hours; adding anti-aflatoxin B with a molar ratio of 10:1 to quantum dots 1 Monoclonal antibody solution, after adjusting the pH of the solution to 7.5 with 1M NaOH solution, react at room temperature for 3 hours; finally add 2% glucosamine to the solution, and further adjust the pH to 4.5 with 1M HCl solution. 18,000rpm (about 29,000g ) Centrifuge at 4°C for 30 min, discard the supernatant, and use 25% glycerol, 0.01% NaN for precipitation 3 0.05mol / L phosphate buffer (pH7.0~7.5) dissolved to obtain free anti-aflatoxin B 1 Monoclonal antibody against water-soluble quantum dots against aflatox...
Embodiment 2
[0033] Example 2 Water-soluble quantum dot anti-ochratoxin monoclonal antibody conjugate and purification process
[0034] Take 5mL of commercial carboxylated water-soluble quantum dots (concentration: 50nmol / L) and mix it with an equal volume of 0.05mol / L borate buffer at pH 6.0; respectively add 1- Ethyl-(3-dimethylaminopropyl)carbodiimide and N-hydroxysulfosuccinimide were reacted at 37°C for 2 hours; anti-ochratoxin was added with a molar ratio of 5:1 to quantum dots Monoclonal antibody solution, after adjusting the pH of the solution to 8.0 with 1M NaOH solution, react at room temperature for 3 hours; finally add 2.5% glucosamine to the solution, and further adjust the pH to 4.5 with 1M HCl solution. 18,000rpm (about 29,000g ) Centrifuge at 4°C for 30 min, discard the supernatant, and use 25% glycerol, 0.01% NaN for precipitation 3 The water-soluble quantum dot anti-ochratoxin monoclonal antibody conjugate without free anti-ochratoxin monoclonal antibody was obtained by ...
Embodiment 3
[0035] Example 3 Water-soluble carboxyl quantum dot anti-zearalenone monoclonal antibody conjugate and purification process
[0036] Take 5mL of commercial carboxylated water-soluble quantum dots (concentration: 50nmol / L) and mix it with an equal volume of 0.05mol / L borate buffer at pH5.5; Base-(3-dimethylaminopropyl)carbodiimide and N-hydroxysulfosuccinimide were reacted at 37°C for 2 hours; anti-zearalen was added with a molar ratio of 3:1 to quantum dots Ketone monoclonal antibody solution, adjust the pH of the solution to 8.0-9.0 with 1M NaOH solution, react at room temperature for 3 hours; finally add 2.5% glucosamine to the solution, and further adjust the pH to 4.5 with 1M HCl solution. 18,000rpm (approx. 29,000 g) at 4°C for 30 min, discard the supernatant, and use 25% glycerol, 0.01% NaN for precipitation 3 The water-soluble quantum dot anti-zearalenone monoclonal antibody conjugate without free anti-zearalenone monoclonal antibody was obtained by dissolving in 0.05m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com