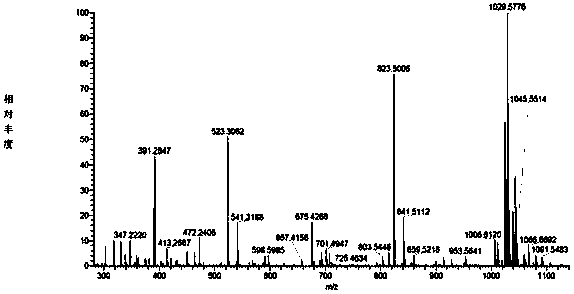
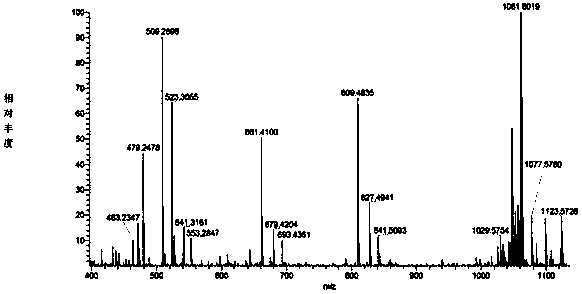
Gopalamicin derivatives and application of same in inhibition of infection by drug-resistant bacteria and drug-resistant mycobacterium tuberculosis
A kind of technology of Mycobacterium tuberculosis, olive leaf element, is applied in the field of fermenting and preparing described natural compound, and marine microbial resource obtains new compound field, can solve the problem that there is no relevant report etc. yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Identification of Streptomyces sp. 7-145
[0046] a) Source of strain: Streptomyces sp. 7-145 was isolated from marine sediments at a depth of about 40m in Heishijiao Bay (38°49'N, 121°34'E) in the Yellow Sea, Dalian, China in our laboratory.
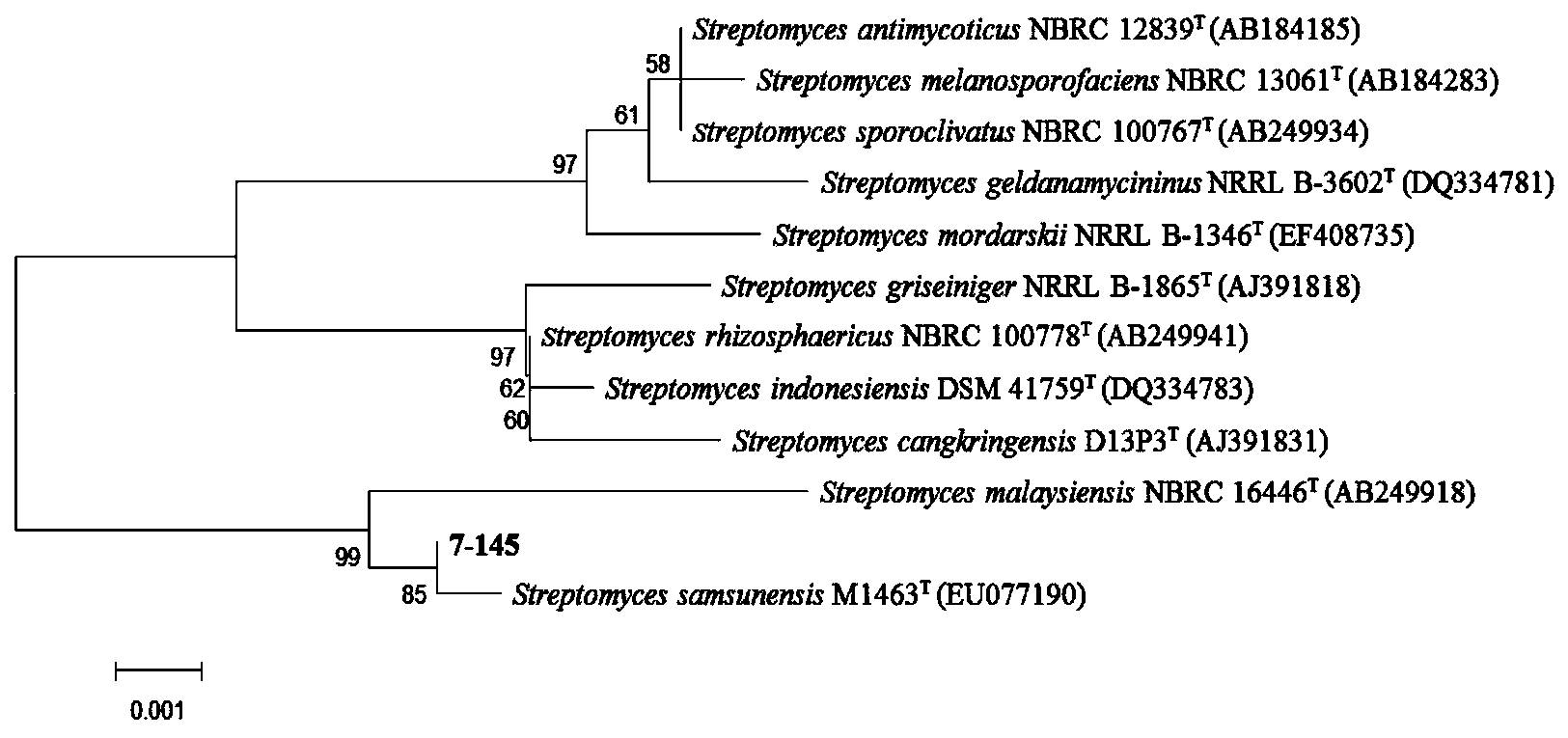
[0047] b) Strain identification: Streptomyces sp. 7-145 was identified based on the conservation of the 16S rRNA gene sequence among microbial species. The genome of Streptomyces sp. 7-145 was extracted, its 16S rRNA gene was amplified by PCR and sequenced, and submitted to the NCBI GenBank database to obtain the accession number JQ782979, and its sequence is shown in SEQ ID NO.1. The results of the 16S rRNA gene comparison of Streptomyces sp. 7-145 showed that the strain was similar to Streptomyces samsunensis M1463 T The similarity is 99.85%. By the proximity method, the phylogenetic evolutionary tree was constructed ( figure 1 ).
[0048] SEQ ID NO.1
[0049] 1 atgaaccggt ttcggccggg gattagtggc gaacgggtga gtaacacgtg ggca...
Embodiment 2
[0073] Fermentation of Streptomyces sp. 7-145
[0074] Take the activated Streptomyces sp. 7-145 plate, pick about 1cm 2 Inoculate the bacterial lawn into 100mL fermentation medium [recipe: 1.0g starch, 2.5g glucose, 1.0g cottonseed cake powder, 0.3g peptone, inorganic salt such as KH 2 PO 4 0.01g, MgSO 4 0.01g, NaCl0.5g, CaCO 3 0.5g, etc., deionized water 100mL], in a 500mL shake flask, 28 ℃, 200r / min shaker culture 120h.
Embodiment 3
[0075] The extraction of fermented liquid and the obtaining of extract
[0076]Streptomyces sp. 7-145 fermentation broth was separated from solid to liquid to obtain filtrate and mycelium; mycelium was extracted by ultrasonic extraction with acetone to obtain acetone extract of mycelium; the filtrate was adsorbed by Amberlite XAD7HP macroporous adsorption resin column , sequentially eluted with water, 50%, 100% acetone; the 50%, 100% acetone eluate part was combined with the acetone extract of mycelium, concentrated under reduced pressure to remove acetone to obtain an aqueous solution, extracted with ethyl acetate; ethyl acetate The extracted part was concentrated under reduced pressure to obtain the fermented liquid extract.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com