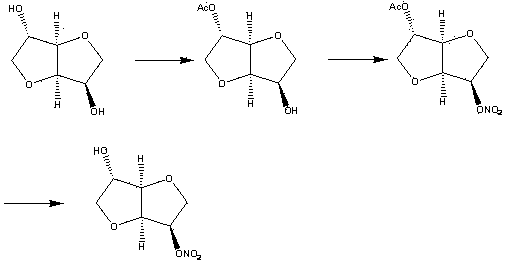
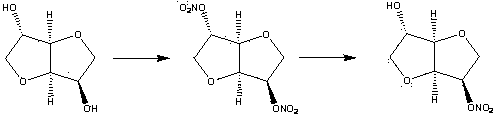
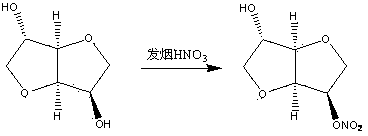
Method for synthesizing and purifying 5-isosorbide mononitrate
A technology of isosorbide dinitrate and purification method, which is applied in the direction of organic chemistry, can solve the problems of incomplete selective reduction, high catalyst cost, and difficulty in purification, and achieve the effects of easy removal of impurities, low price, and short reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
[0032] The synthesis and purification method of present embodiment 5-isosorbide mononitrate are as follows:
[0033] 1. In a 100 mL dry three-necked flask, add acetic acid (3.6 g, 60 mmol) and acetic anhydride (6.12 g, 60 mmol), keep the internal temperature of the reaction at 0±1 °C, and start adding concentrated nitric acid (5.82 g , 60 mmol), and the dropping time was 30 min to prepare a nitrating reagent (concentrated nitric acid was activated by acetic acid and acetic anhydride). After the dropwise addition, keep the reaction at 0±1°C for 30 min.
[0034] Slowly add the prepared nitrating reagent dropwise into a 250 mL single-necked bottle containing isosorbide (8.76 g, 60 mmol), 10 mL of acetic acid and 5 mL of acetic anhydride (activated concentrated nitric acid and isosorbide in the nitrating reagent The reaction occurs, the acetic acid and acetic anhydride added here are used as solvents), the reaction temperature is kept at 12±2 °C, and the dropwise addition time is...
Embodiment 2
[0039] The synthesis and purification method of present embodiment 5-isosorbide mononitrate are as follows:
[0040] 1. Add acetic acid (3.6 g, 60 mmol) and acetic anhydride (6.12 g, 60 mmol) into a 100 mL dry three-necked flask, keep the internal temperature of the reaction at -5±1 °C, and start adding concentrated nitric acid (6.98 g, 72 mmol), the dropping time was 30 min. After the dropwise addition, keep at -5±1°C for 45 min.
[0041] Slowly add the prepared nitration reagent dropwise into a 250 mL single-necked bottle filled with isosorbide (8.76 g, 60 mmol), 10 mL of acetic acid and 5 mL of acetic anhydride, keeping the reaction temperature at 15±2 °C, and the dropping time for 1 h. After the dropwise addition, keep the temperature at 15±2°C for 2 h.
[0042] 2. Add 100 mL of water to the reaction solution to quench the reaction. The reaction solution was left to stand at 0 °C for 3 h to crystallize, and a white solid precipitated out. It was filtered while cold, an...
Embodiment 3
[0046] The synthesis and purification method of present embodiment 5-isosorbide mononitrate are as follows:
[0047] 1. In a 100 mL dry three-necked flask, add acetic acid (3.6 g, 60 mmol) and acetic anhydride (6.12 g, 60 mmol), keep the internal temperature of the reaction at 0±1 °C, and start adding concentrated nitric acid (6.98 g , 72 mmol), the dropping time was 30 min. After the dropwise addition, keep the reaction at 0±1°C for 30 min.
[0048] Slowly add the prepared nitration reagent dropwise into a 250 mL single-necked bottle filled with isosorbide (8.76 g, 60 mmol), 10 mL of acetic acid and 5 mL of acetic anhydride, keeping the reaction temperature at 12±2 °C, and the time for dropping for 1 h. After the dropwise addition, keep the temperature at 12±2°C for 2 h.
[0049] 2. Add 100 mL of water to the reaction solution to quench the reaction. The reaction solution was left to stand at 0 °C for 3 h to crystallize, and a white solid precipitated out. It was filtered...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com