Method for preparing hexagonal boron nitride nanosheets by chemical stripping
A hexagonal boron nitride, nanosheet technology, applied in chemical instruments and methods, nanotechnology for materials and surface science, nanotechnology, etc. Small size and other problems, to achieve the effect of being conducive to large-scale production, low cost, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (1) Mix 1g of hexagonal boron nitride powder with 100ml of sulfuric acid and stir evenly, then add 0.5g of potassium permanganate (the mass ratio of potassium permanganate to boron nitride is 1:2), and continue to stir and react to form a mixture ;
[0016] (2) Put the mixture in an ice bath, stir and react for 12 hours; then add hydrogen peroxide dropwise to remove the remaining potassium permanganate;
[0017] (3) Finally, the reaction product was washed with water to a pH value of 6-8, then dissolved in absolute ethanol for separation, and the unstripped boron nitride powder was removed to obtain boron nitride nanosheets.
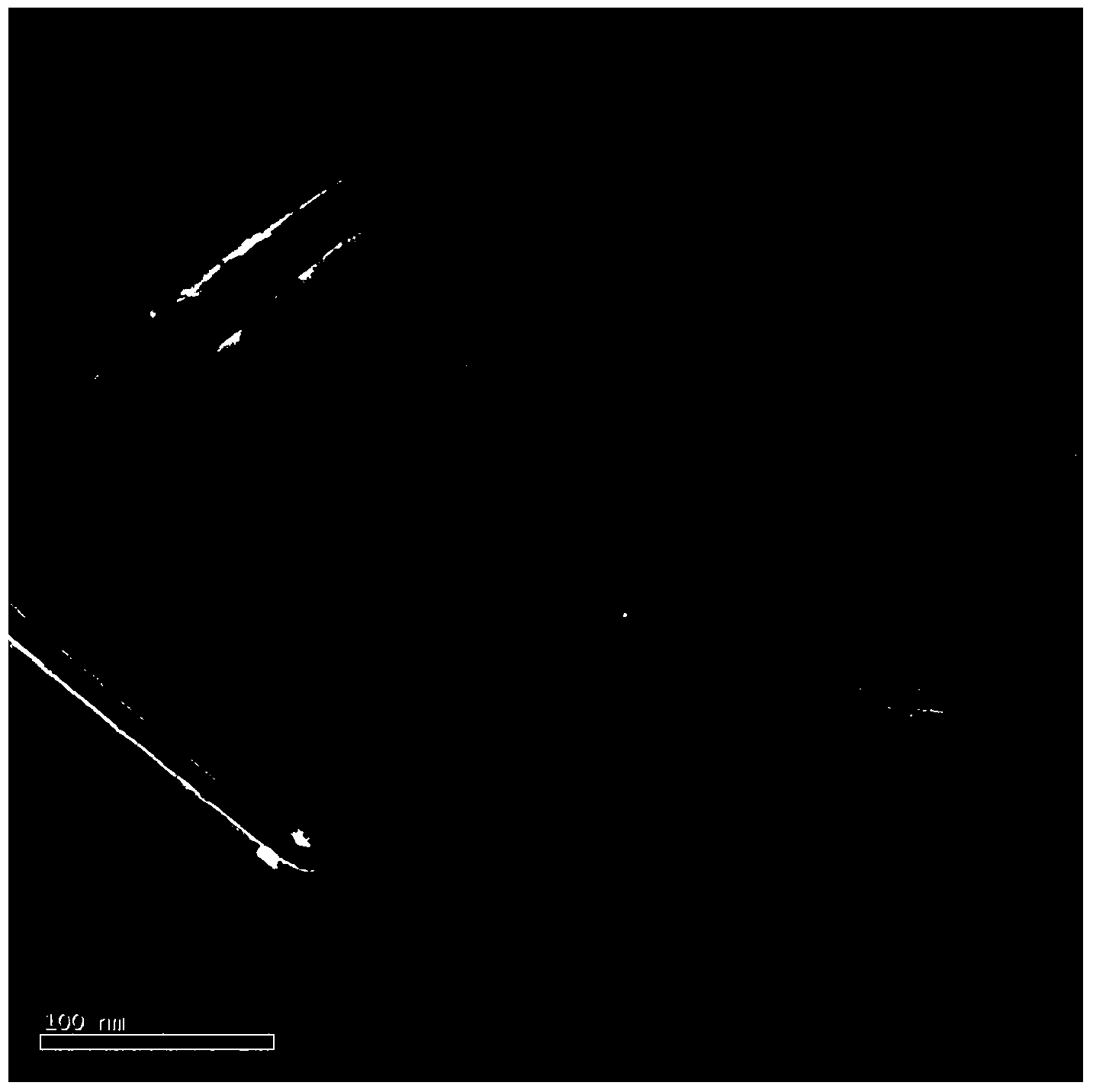
[0018] figure 1 The scanning electron micrographs of the boron nitride nanosheets prepared in this example are given.
[0019] figure 2 The transmission electron micrographs of the boron nitride nanosheets prepared in this example are given.
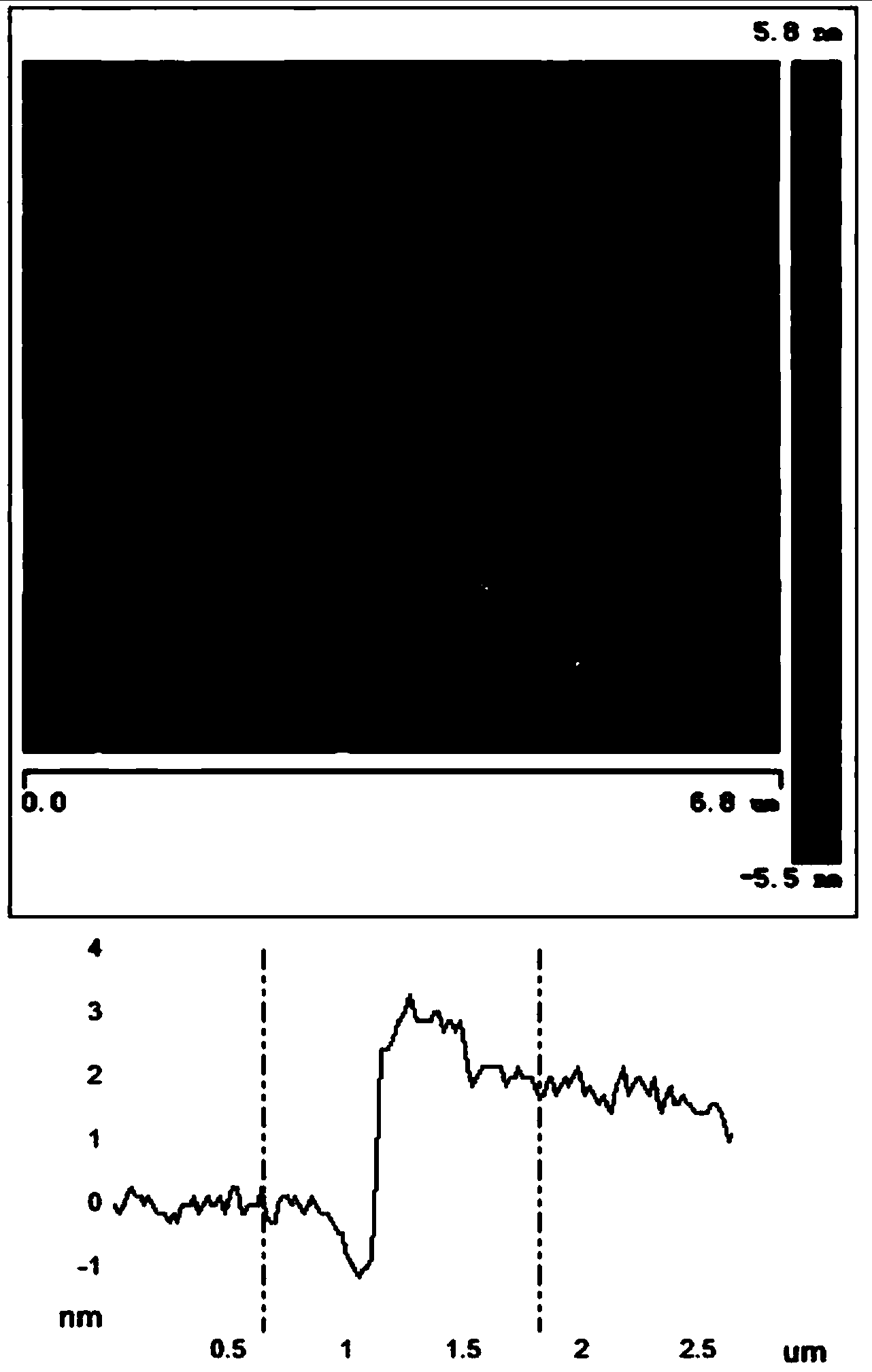
[0020] image 3 The atomic force microscope photos and thicknesses of the boron nitride nanosheets ...
Embodiment 2
[0022] (1) Mix 3g of hexagonal boron nitride powder and 100ml of nitric acid and stir evenly, then add 3g of potassium permanganate (the mass ratio of potassium permanganate to boron nitride is 1:1), continue to stir and react to make a mixture;
[0023] (2) Put the mixture in an ice bath, stir and react for 10 hours; then add hydrogen peroxide dropwise to remove the remaining potassium permanganate;
[0024] (3) Finally, the reaction product was washed with water to a pH value of 6-8, then dissolved in absolute ethanol for separation, and the unstripped boron nitride powder was removed to obtain boron nitride nanosheets.
Embodiment 3
[0026] (1) Mix 5g of hexagonal boron nitride powder with 100ml of sulfuric acid and stir evenly, then add 0.5g of potassium permanganate (the mass ratio of potassium permanganate to boron nitride is 1:10), continue to stir and react to make a mixture ;
[0027] (2) Put the mixture in an ice bath, stir and react for 24 hours; then add hydrogen peroxide dropwise to remove the remaining potassium permanganate;
[0028] (3) Finally, the reaction product was washed with water to a pH value of 6-8, then dissolved in absolute ethanol for separation, and the unstripped boron nitride powder was removed to obtain boron nitride nanosheets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com