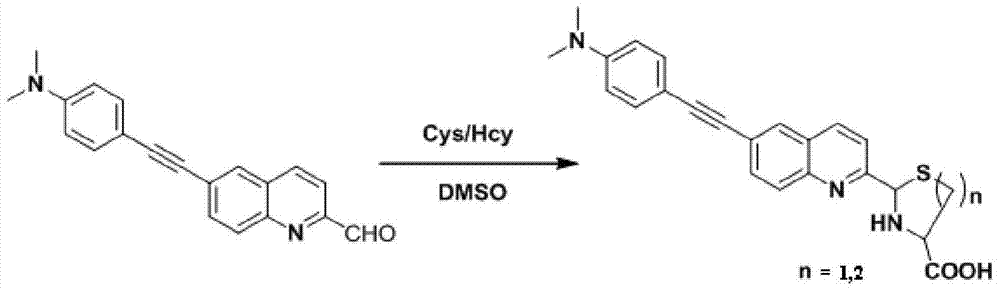
Two-photon fluorescent probe as well as preparation method and application thereof
A two-photon fluorescence and probe technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem of low sensitivity detection concentration, achieve low detection concentration, specific selectivity, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of fluorescent probe molecule NQ
[0029] 1. Synthesis of intermediate 1
[0030] Weigh 15g (68.49mmol) of p-iodoaniline and 100mL of 6mol / L hydrochloric acid in a three-neck flask, install a condenser, heat to 105°C, until the solid is completely dissolved, slowly add 12g (171mmol) of crotonaldehyde dropwise, and track with TLC After the reaction of the raw materials is completed, stop the reaction, cool to room temperature, pour the reaction solution into 200mL water, extract with ethyl acetate (100mL×2) to remove unreacted crotonaldehyde, and neutralize the aqueous solution with ammonia water to a pH value of 8. Extract with ethyl acetate (50mL×2), combine the organic phases, dry the organic phase with anhydrous sodium sulfate, and spin dry to obtain the crude product, which is reconstituted in a mixed solvent of ethyl acetate and petroleum ether (volume ratio 1:20). Crystallization gave 14.45 g (53.72 mmol) of intermediate 1, with a yiel...
Embodiment 2
[0038] Example 2: Two-photon testing of fluorescent probe molecules
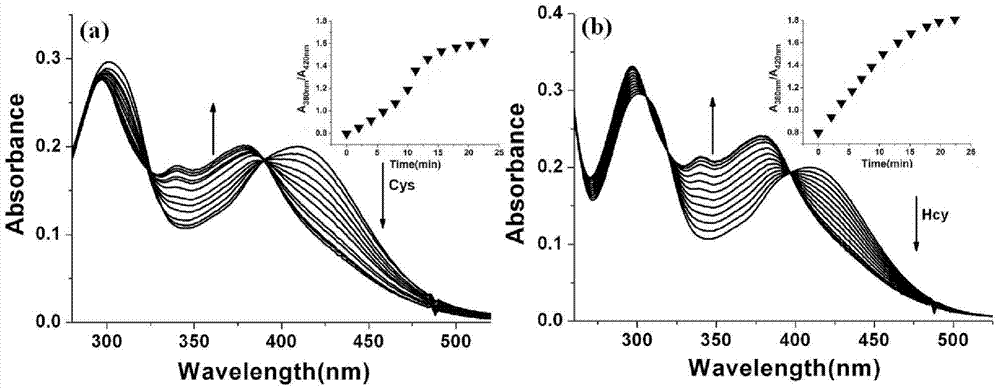
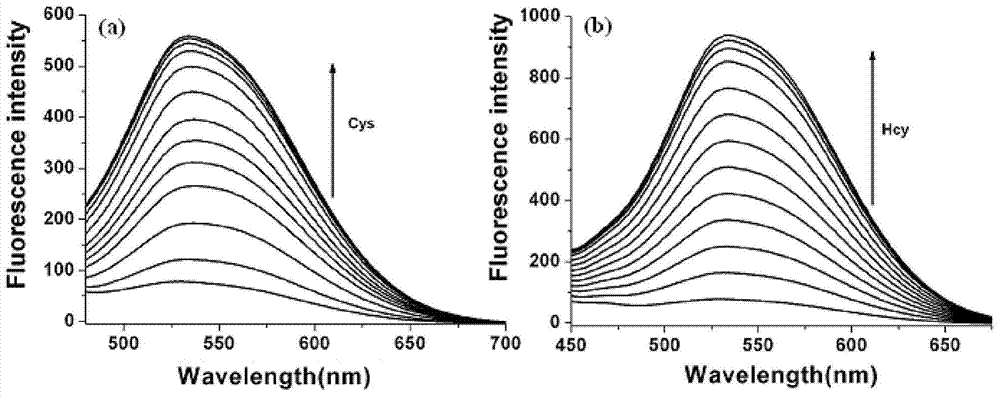
[0039] Using the two-photon measurement technique, the two-photon absorption cross section of the fluorescent probe molecule (NQ) and the fluorescent probe molecule reacted with cysteine (MQ-Cys) was tested, from Figure 4 It can be seen that the maximum absorption cross-sections of fluorescent probe molecules reacted with cysteine or homocysteine (NQ-Cys or NQ-Hcy) are 587 and 693 GM respectively, and the two-photon excitation wavelengths are both at 800 nm. Example 3: Cytotoxicity Test
Embodiment 3
[0040] The MTT (3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide) experiment was based on the reported articles to do some cytotoxicity tests. Add 0, 10, 30, and 80 μM fluorescent probe molecules to the same batch of cells, and the conditions are at 37 ° C, containing 5% CO 2 According to the formula of cell viability: cell viability %=OD570 (sample) / OD570 (control group) × 100, the cell viability can be calculated ( Figure 5 ). From Figure 5 We can see that when the concentration is 10 μM, the cell survival rate is still about 98%, which shows that the fluorescent probe molecule of the present invention has no toxic effect on cells, so it can be used for cell detection and tracking amino acid small molecules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com