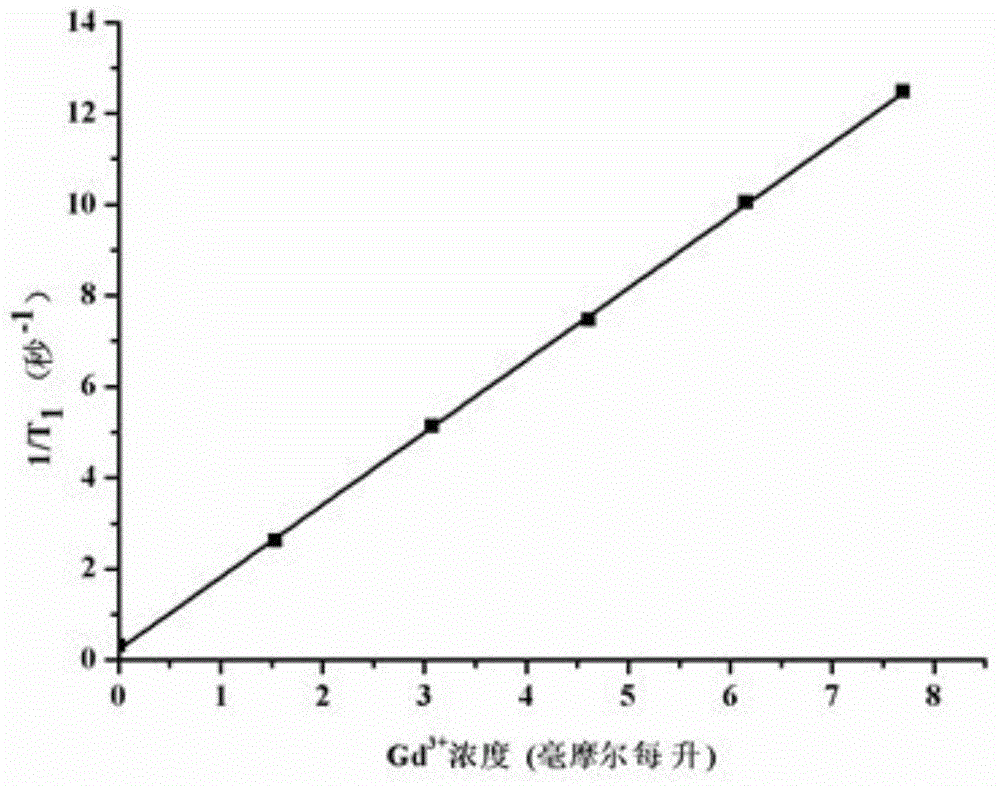
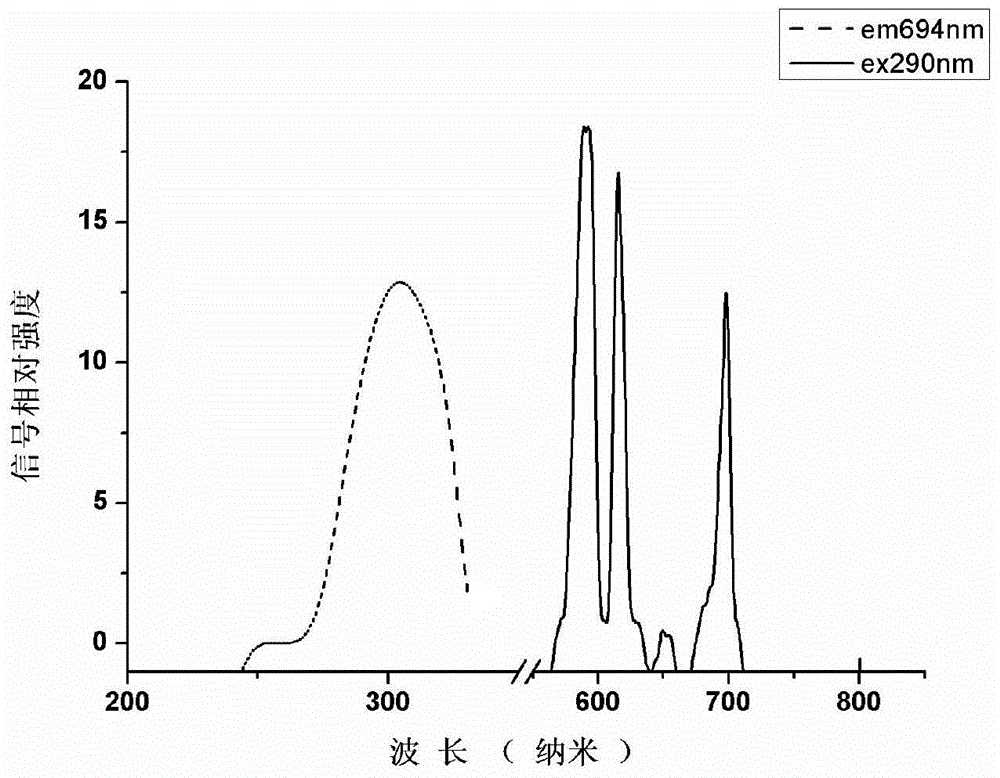
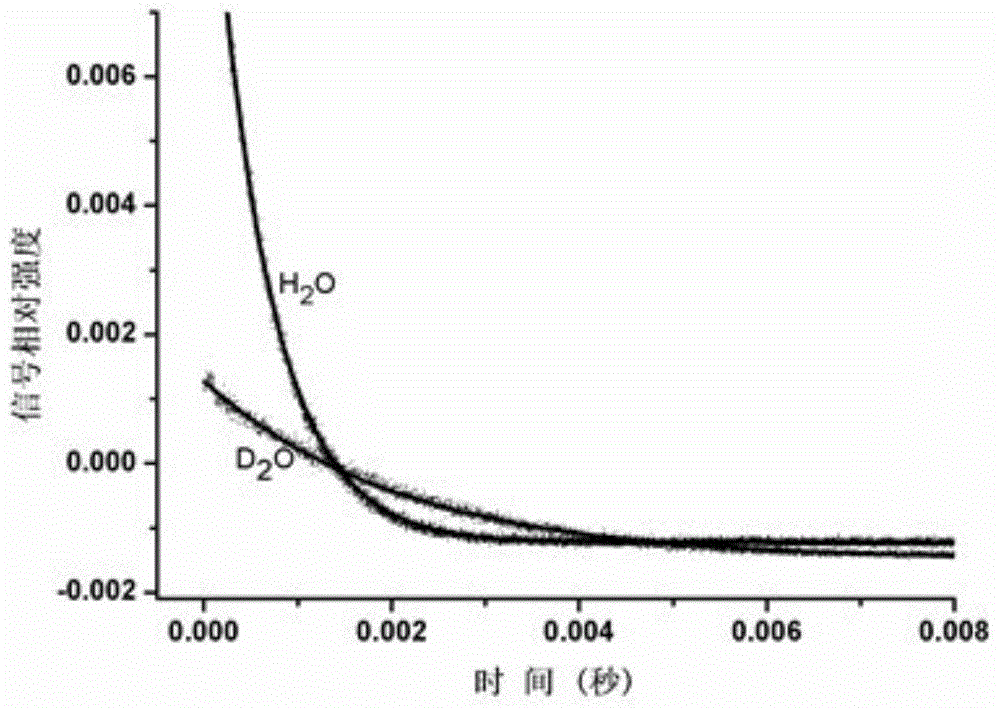
Non-ionic multi-nuclear magnetic resonance imaging contrast medium taking tetrabenzoylmethane as interconnect and preparation method thereof
A technology of nuclear magnetic resonance imaging and tetrabenzoylmethane, which is applied in the direction of nuclear magnetic resonance/magnetic resonance imaging contrast agents, chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, etc., can solve the problem of long residence time, relaxation High relaxation efficiency, slow metabolism, etc., to achieve the effect of reducing the flexibility of the linker, improving the relaxation efficiency, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Step 1: Synthesis of tetrakis-(4-(bromoacetyl)phenyl)methane
[0051] Under nitrogen protection, dissolve 0.8g tetraphenylmethane in 30mLCS 2 In, add 2.5g 2-bromoacetyl bromide, then add 1.7gAlCl 3 , reflux, react at 50°C for 28h, pour off CS 2 , add 25g ice water, 7mL concentrated hydrochloric acid, 25mL dichloromethane and stir until the black solid is completely dissolved; layered extraction, add water and saturated sodium chloride to wash, dry over anhydrous sodium sulfate, pass through a silica gel column, the mobile phase is dichloromethane: n-Hexane = 20:1, and finally 1.4 g of white foamy solid tetrakis-(4-(bromoacetyl)phenyl)methane was obtained.
[0052] Step 2: Tetrakis(4-(2-tris-(4,7,10-triacetate tert-butylcarboethoxy)1,4,7,10-tetraazacyclododecylacetyl)phenyl) Methane synthesis
[0053] Add 1.6g of tri-tert-butyl 2,2',2''-(1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triethyl to a 20mL reaction tube Ester · Hydrogen bromide hydrochloride ( t Bu-DO3A·HB...
Embodiment 2
[0061] Step 1: Synthesis of tetrakis-(4-(bromoacetyl)phenyl)methane
[0062] Under nitrogen protection, dissolve 1.6g tetraphenylmethane in 60mLCS 2 In, add 5g of 2-bromoacetyl bromide, and finally add 3.4g of AlCl 3 , reflux, react at 50°C for 25h, pour off the CS 2 , add 50g ice water, 13mL concentrated hydrochloric acid, 50mL dichloromethane and stir until the black solid is completely dissolved; layered extraction, add water and saturated sodium chloride to wash, dry over anhydrous sodium sulfate, pass through a silica gel column, the mobile phase is dichloromethane: n-Hexane=20:1, and finally 2.6 g of white foamy solid tetrakis-(4-(bromoacetyl)phenyl)methane was obtained.
[0063]Step 2: Tetrakis(4-(2-tris-(4,7,10-triacetate tert-butylcarboethoxy)1,4,7,10-tetraazacyclododecylacetyl)phenyl) Methane synthesis
[0064] Add 3.2 g of tri-tert-butyl 2,2',2''-(1,4,7,10-tetraazacyclododecane-1,4,7-triyl) tris to a 50 mL round bottom flask Acetate · hydrogen bromide hydrochlo...
Embodiment 3
[0078] Step 1: Synthesis of tetrakis-(4-(bromoacetyl)phenyl)methane
[0079] Under nitrogen protection, dissolve 2g tetraphenylmethane in 75mLCS 2 In, add 6.3g of 2-bromoacetyl bromide, and finally add 4.3g of AlCl 3 , reflux, react at 55°C for 24h, pour off the CS 2 , add 65g ice water, 16mL concentrated hydrochloric acid, 65mL dichloromethane and stir until the black solid is completely dissolved; layered extraction, add water and saturated sodium chloride to wash, dry over anhydrous sodium sulfate, pass through a silica gel column, and the mobile phase is dichloromethane: n-Hexane=20:1, and finally 3.4 g of white foamy solid tetrakis-(4-(bromoacetyl)phenyl)methane was obtained.
[0080] Step 2: Tetrakis(4-(2-tris-(4,7,10-triacetate tert-butylcarboethoxy)1,4,7,10-tetraazacyclododecylacetyl)phenyl) Methane synthesis
[0081] Add 3.4 g of tri-tert-butyl 2,2',2''-(1,4,7,10-tetraazacyclododecane-1,4,7-triyl) tris to a 50 mL round bottom flask Acetate · hydrogen bromide hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com