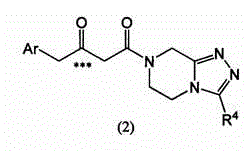
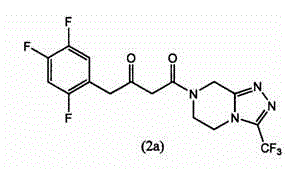
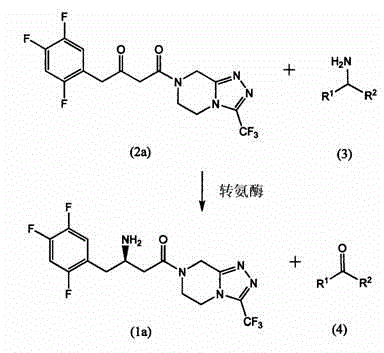
Immobilized transaminases and process for making and using immobilized transaminase
A technology for immobilizing transaminase and transaminase is applied in the fields of immobilized transaminase and production and use of immobilized transaminase, and can solve the problems of inability to reuse catalyst, discarding, lack of stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0187] Example 1-5: Immobilization of Transaminase
[0188] Five different resins as shown in Table 2 were evaluated.
[0189] Table 2
[0190] Example Resin combination 1 SEPABEADS EC-EP Polymethacrylate / epoxide 2 SEPABEADS EC-HFA / S Polymethacrylate / amino epoxide 3 SEPABEADS EXA252 Styrene / DVB copolymer 4 SEPABEADS EXE119 Polymethacrylate / epoxide 5 SEPABEADS EXE120 Polymethacrylate / octadecyl
[0191] A 25 g / L solution of SEQ ID NO: 110 with 1 g / L PLP (pyrodoxial-5-phosphate) in 100 mM potassium phosphate buffer (pH 7.5) was prepared. Incubate 1 g of each resin with 5 mL of enzyme solution in a shaker at room temperature overnight. The resin was filtered from the transaminase solution and rinsed 5 times with 5 mL of 100 mM potassium phosphate buffer (pH 7.5). The resin performance in the following transaminations was then evaluated compared to the freeze-dried enzyme preparation:
[0192] .
[0193] The immobilized transaminase preparation exhibits superior specific activity co...
Embodiment 6
[0194] Example 6: Evaluation of the drying method of SEPABEADS EXE120 resin and confirmation of the activity of the immobilized enzyme in a 100% organic solvent system
[0195] The immobilized enzyme (SEQ ID NO: 110 / SEPABEADS EXE120 resin) was dried under vacuum and purged with nitrogen to remove water from the outer surface of the immobilized enzyme resin. The immobilized preparation is stirred while drying to allow a uniform moisture content throughout the immobilized enzyme bed, and to prevent any part of the immobilized enzyme preparation from over-drying or insufficiently drying. 100 mg of ketoamide substrate was dissolved in 1 mL of water-saturated IPAc (isopropyl acetate). Add 40 uL IPM (isopropylamine) to the reaction solution. 100 mg of dried immobilized transaminase (SEQ ID NO: 110 / SEPABEADS EXE120 resin) was added to the reaction. The reaction was stirred at 1000 rpm in a Thermomixer at 50°C. After 70 hours, a sample was taken and the conversion rate and ee were det...
Embodiment 7
[0196] Example 7: Plug flow reaction (PFR) method for the production of sitagliptin in IPAc
[0197] Prepare 10 mL of ketonamide substrate solution (100g / L-400g / L ketone, 40uL / mL-160uL / mL isopropylamine in IPAc). 0.75 g of immobilized transaminase (SEQ ID NO: 110 / SEPABEADS EXE120 resin) slurry was packed in the column using IPAc under gravity. The substrate solution was added to the column via a syringe pump, which was layered at 50°C. Set the flow rate to 0.1 mL / h.
[0198] In steady state, sitagliptin amine was observed at the exit of the column (> 99% ee) 85-90% conversion rate.
[0199]
[0200]
[0201]
[0202]
[0203]
[0204]
[0205]
[0206]
[0207]
[0208]
[0209]
[0210]
[0211]
[0212]
[0213]
[0214]
[0215]
[0216]
[0217]
[0218]
[0219]
[0220]
[0221]
[0222]
[0223]
[0224]
[0225]
[0226]
[0227]
[0228]
[0229]
[0230]
[0231]
[0232]
[0233]
[0234]
[0235]
[0236]
[0237]
[0238]
[0239]
[0240]
[0241]
[0242]
[0243]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com