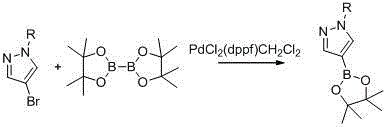
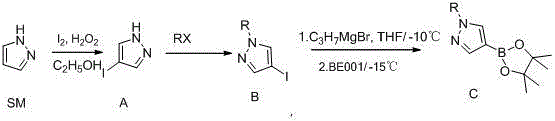
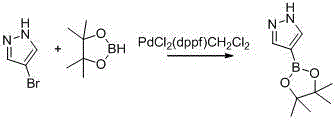Synthetic method of 1-alkylpyrazole-4-boronic acid pinacol ester
A technology of pinacol ester and alkylpyrazole, which is applied in the field of organic compound synthesis and achieves the effects of simple synthesis route, low cost of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for preparing 1-alkylpyrazole-4-boronic acid pinacol ester, 1-methylpyrazole-4-boronic acid pinacol ester is an example:
[0027] The preparation of the first step 4-iodopyrazole:
[0028] The raw material pyrazole (10g) and ethanol (10g) are stirred evenly, and iodine (22.4g) is added in batches, and the temperature rises (20°C-30°C), and 30% hydrogen peroxide (16.7g) is added dropwise, and the temperature is not higher than 70 After the dropwise addition was completed and stirred for 1 hour, TLC tracked the end of the reaction, added 10 g of saturated aqueous sodium bisulfite solution, stirred for 1 hour, filtered to obtain a white solid, washed the filter cake with 20 g of water, concentrated the filtrate, and solids were precipitated, re-filtered and washed, combined and filtered The cake was dried, weighing 25.7g, GC purity: 99%, yield 90%;
[0029] The preparation of second step 1-methyl-4-iodopyrazole:
[0030] Stir 4-iodopyrazole (20g) and ethanol (10...
Embodiment 2
[0034] A method for preparing 1-alkylpyrazole-4-boronic acid pinacol ester, 1-methylpyrazole-4-boronic acid pinacol ester is an example:
[0035] The preparation of the first step 4-iodopyrazole:
[0036] The raw material pyrazole (10g) and ethanol (10g) are stirred evenly, and iodine (22.4g) is added in batches, there is a phenomenon of temperature rise (20°C-30°C), and hydrogen peroxide (16.7g) is added dropwise, and the temperature is controlled not to exceed 70°C , the dropwise addition was completed and stirred for 1 hour, followed by TLC for the completion of the reaction, adding 10 g of saturated aqueous sodium bisulfite solution, stirring for 1 hour, filtering to obtain a white solid, washing the filter cake with 20 g of water, concentrating the filtrate, and solids were precipitated, re-filtering and washing, and combining the filter cake Drying, weighing 25.7g, GC purity: 99%, yield 90%;
[0037] The preparation of second step 1-methyl-4-iodopyrazole:
[0038] Stir...
Embodiment 3
[0043] A method for preparing 1-alkylpyrazole-4-boronic acid pinacol ester, 1-isopropylpyrazole-4-boronic acid pinacol ester is an example:
[0044] The preparation of the first step 4-iodopyrazole:
[0045] The raw material pyrazole (10g) and ethanol (10g) are stirred evenly, and iodine (18.6g) is added in batches, and the temperature rises (20°C-30°C), and 30% hydrogen peroxide (16.7g) is added dropwise, and the temperature is controlled not to exceed 70 degrees, stirring for 1 hour after the dropwise addition, TLC tracking the end of the reaction, adding 10g of saturated sodium bisulfite aqueous solution, stirring for 1 hour, filtering to obtain a white solid, washing the filter cake with 20g of water, concentrating the filtrate, solids precipitated, re-filtering and washing, and combining Filter cake was dried, weighed 22.8g, GC purity: 99%, yield 80%;
[0046] The preparation of second step 1-isopropyl-4-iodopyrazole:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com