Gel polymer electrolyte and preparation method thereof
A gel polymer and electrolyte technology, applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, electrolyte immobilization/gelation, etc., can solve the problem of low conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
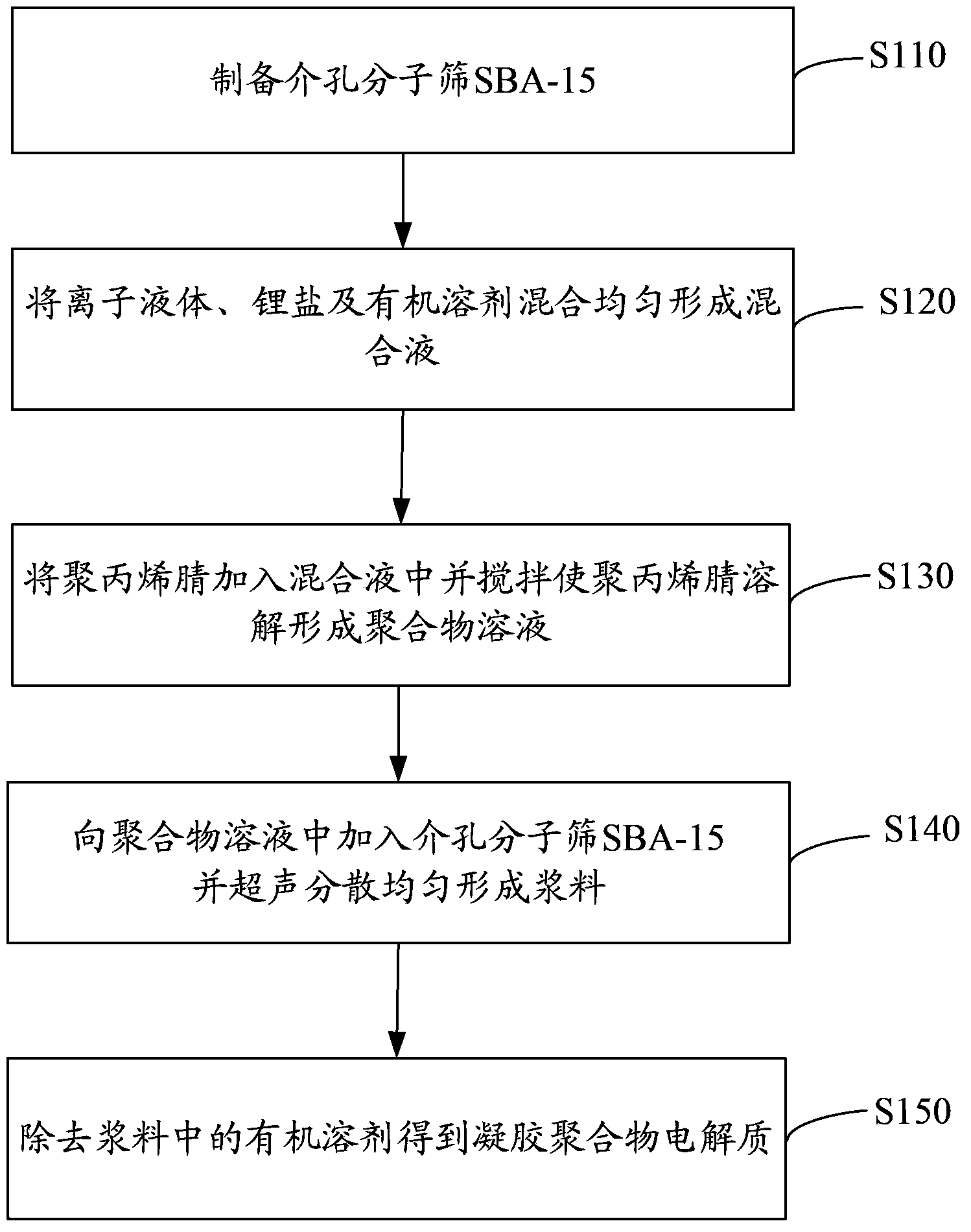
[0033] See figure 1 , The preparation method of the above-mentioned gel polymer electrolyte includes the following steps:
[0034] Step S110, preparing the mesoporous molecular sieve SBA-15.
[0035] The preparation of mesoporous molecular sieve SBA-15 includes the following steps:
[0036] Step S111. Dissolve (1,2-ethylene glycol)-propylene glycol-(1,2-ethylene glycol) block copolymer in hydrochloric acid, then add ethyl orthosilicate dropwise, and stir for 24 hours The above forms a homogeneous solution, in which the mass ratio of (1,2-ethylene glycol)-propylene glycol-(1,2-ethylene glycol) block copolymer to ethyl orthosilicate is 1:2.
[0037] Preferably, the molecular weight of the (1,2-ethylene glycol)-propylene glycol-(1,2-ethylene glycol) block copolymer is 2000 to 10000, preferably 5800.
[0038] Preferably, the mass concentration of hydrochloric acid is 0.1%-10%.
[0039] Preferably, the (1,2-ethylene glycol)-propylene glycol-(1,2-ethylene glycol) block copolymer is dissolved...
Embodiment 1
[0058] The preparation method of the gel polymer electrolyte of this embodiment includes the following steps:
[0059] (1) Preparation of mesoporous molecular sieve SBA-15:
[0060] 10g of triblock copolymer P123 (triblock copolymer poly(1,2-ethylene glycol)-block-poly(propylene glycol)-block-poly(1,2-ethylene glycol) , Average molecular weight 2000) was dissolved in deionized water (20mL) and 20% hydrochloric acid solution (60mL) and stirred and mixed well. Slowly add ethyl orthosilicate (20g) dropwise, continue stirring for more than 24h to form a homogeneous solution, add acetic acid (5g), and continue stirring at 40°C for 24h. Finally, the reaction solution was transferred into an autoclave with a polytetrafluoroethylene substrate, and then crystallized at 100°C for 48 hours, cooled, and the resulting crystal product was suction filtered, washed, and dried at 100°C for 24 hours. Then, it is calcined at 550°C for 4-12h to remove the triblock copolymer, and the mesoporous molec...
Embodiment 2
[0064] The preparation method of the gel polymer electrolyte of this embodiment includes the following steps:
[0065] (1) Preparation of mesoporous molecular sieve SBA-15:
[0066] 10g of triblock copolymer P123 (triblock copolymer poly(1,2-ethylene glycol)-block-poly(propylene glycol)-block-poly(1,2-ethylene glycol) , Average molecular weight 5800) was dissolved in deionized water (20mL) and 30% hydrochloric acid solution (60mL) and stirred and mixed well. Slowly add ethyl orthosilicate (20g) dropwise and continue stirring for more than 24h. After a homogeneous solution was formed, acetic acid (5g) was added, and stirring was continued for 24h at 40°C. Finally, the reaction solution was transferred into an autoclave with a polytetrafluoroethylene substrate, and then crystallized at 100°C for 48 hours, cooled, and the resulting crystal product was suction filtered, washed, and dried at 100°C for 24 hours. Then, it is calcined at 550°C for 4-12h to remove the triblock copolymer,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com