Short peptide, preparation method thereof, and applications of the short peptide in diagnosis or treatment of apoA-I related diseases
A technique for simulating peptides, ac-d-w-f-k-a-f-y-d-k-v-a-e-k-f-k-e-a-f-nh2, applied in the field of peptides, can solve problems such as increasing the risk of atherosclerosis, achieve the level of reducing and antioxidative PL, facilitate automatic operation, and have high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 L-4F
[0023] The L-4F of this embodiment is prepared by solid-phase synthesis, and its specific steps are as follows:
[0024] The short peptide was synthesized using a PS3 small-scale fully automatic peptide synthesizer (PS3 Protein Technologies, Woburn, MA). The L-form FMOC amino acid was coupled to an amide resin, condensed with HBTU and NMM (N-methylmorpholine), and acetylated at the N-terminus with acetic anhydride. Add 1% anisole (dichloromethane anisole), 0.1% mercaptoethanol and tryptophan (20% by weight of the peptide resin), the short peptide is eluted from the column, and then purified by reverse-phase high-performance liquid chromatography . The chromatographic column is a VYDAC C-4 column (22mm×25cm, 10μm), the eluent is acetonitrile containing 0.1% TFA (trifluoroacetic acid), the elution gradient is 0-66min, 25%-85%, and the flow rate is 4.8ml / min. The purity of the peptide was analyzed by reverse-phase high-performanc...
Embodiment 2
[0025] Example 2 Inhibitory effect of L-4F on lupus-like phenotype of autoimmune disease
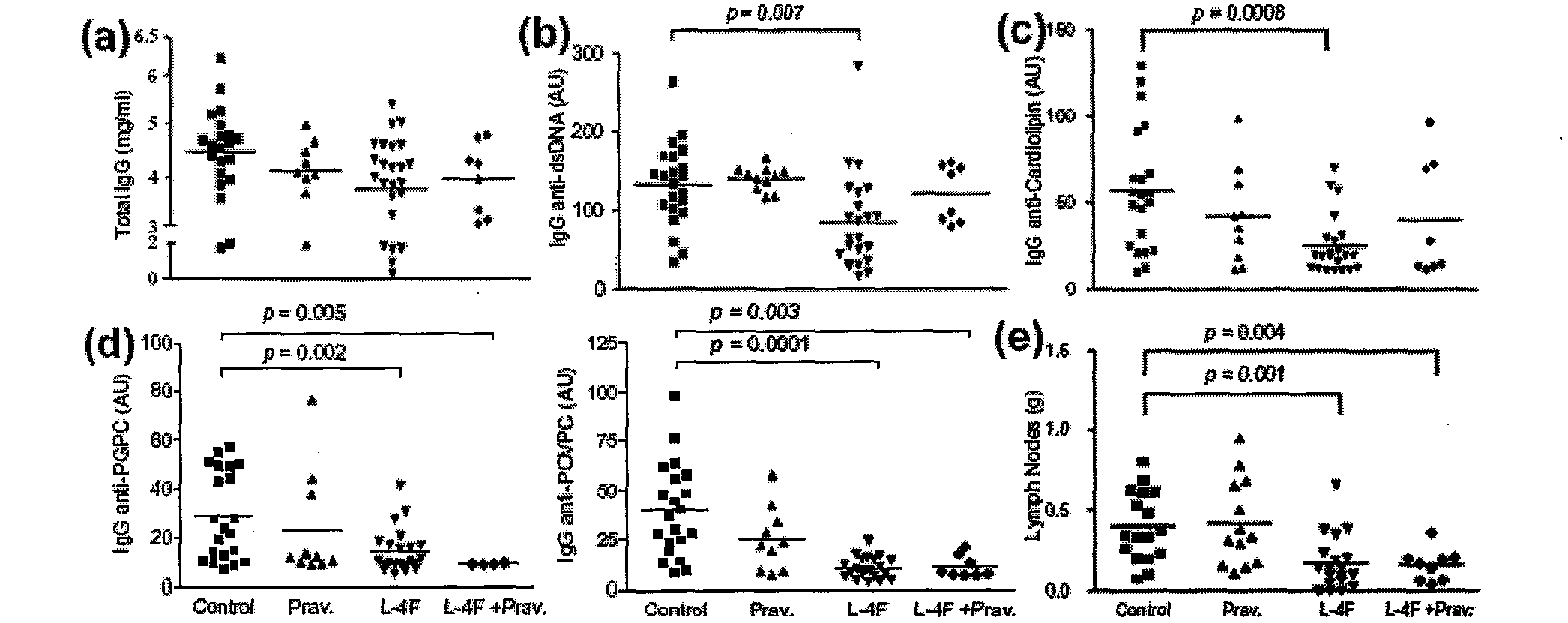
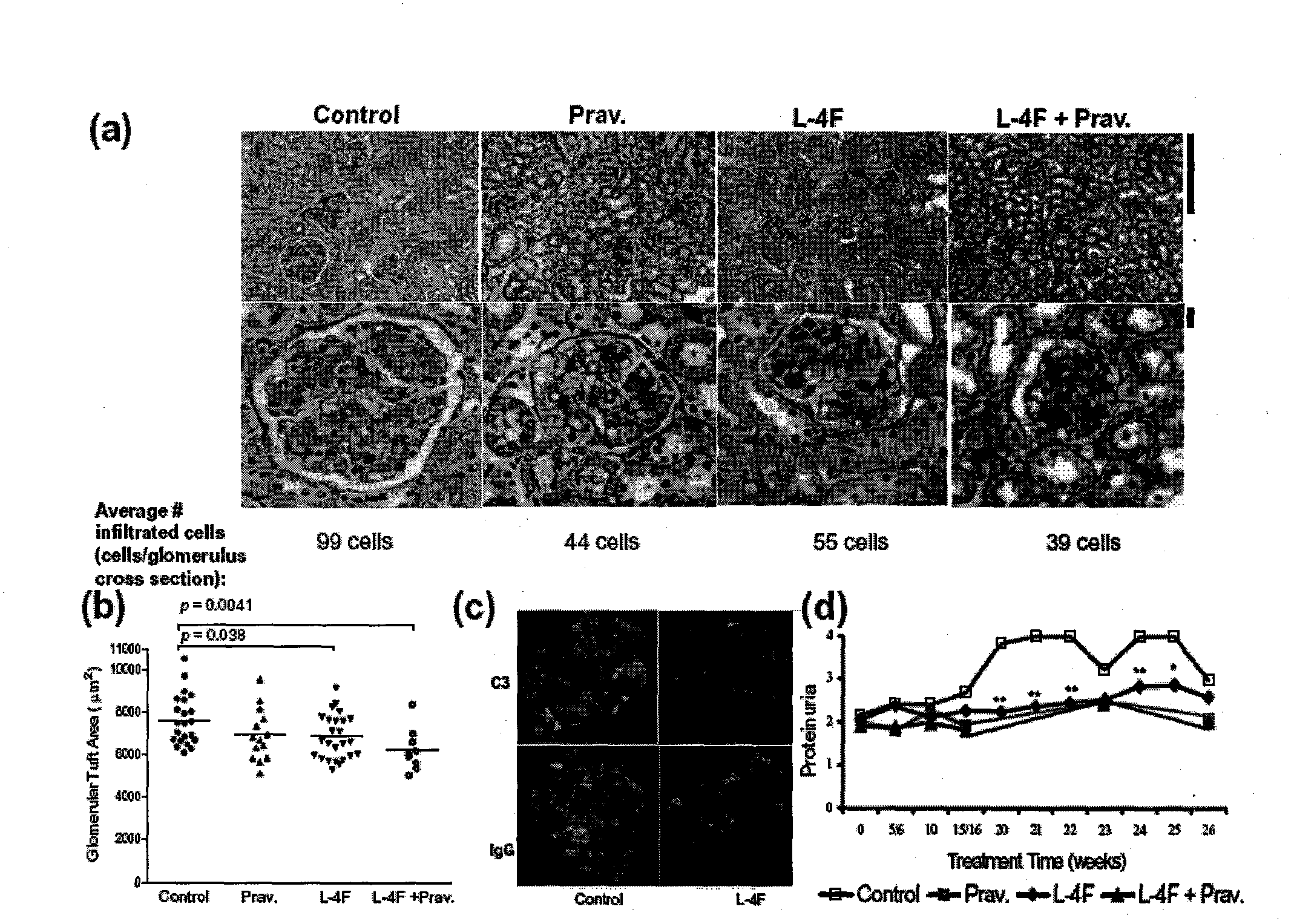
[0026] 26-27 week administration: 1) pravastatin, 2) L-4F, 3) L-4F+pravastatin, 4) blank control. Compared with the mice in the blank control group, the L4F or L-4F+pravastatin group administration group showed that the appearance of lupus-like autoimmunity was improved, and the lymph nodes were significantly reduced (0.17±0.17g: 0.40±0.22g and 0.16±0.10g : 0.40±0.22g; p=0.0012, 0.0007). However, there was no significant change in spleen size and splenocyte number in the treatment group compared with the blank control group, in which B cells, CD4+, CD8+ T cells, NK, CD11c, and CD11b cells were determined by multicolor flow analysis (data not shown). listed).
[0027] In the test, the total IgG levels of the four groups were not much different, and it is generally believed that there is no inhibitory effect on immunity ( figure 1C). Serum IgG anti-double-stranded DNA antibody in the L...
Embodiment 3
[0029] Example 3 L-4F Treatment Little Alters Circulating Serum Levels of Inflammatory Chemokines and Cytokines
[0030] To explore potential biomarker changes among treatment groups, we analyzed female apoE using a Luminex-based beadarray - / - Fas - / - 68 inflammatory chemokines and cytokines in mice. The results showed that compared with the control group, the levels of tissue damage and inflammation in the L-4F group decreased: including CRP (C-reactive protein), fibrinogen, TNF-α (tumor necrosis factor α), CCL12 (mono nuclear cell chemoattractant protein 5 [MCP-5]). Multi-sample (54 detected antigens) Bonferroni correction showed that whether or not pravastatin was used, serum IL-10 (interleukin 10), CCL9 (macrophage inflammatory protein 1γ [MIP-1γ]), VCAM-1 (vascular The level of cell adhesion molecule 1) was significantly lower in the L-4F group than in the control group, and the level of circulating CCL19 (macrophage inflammatory protein 3γ [MIP-3γ]) was also significa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com