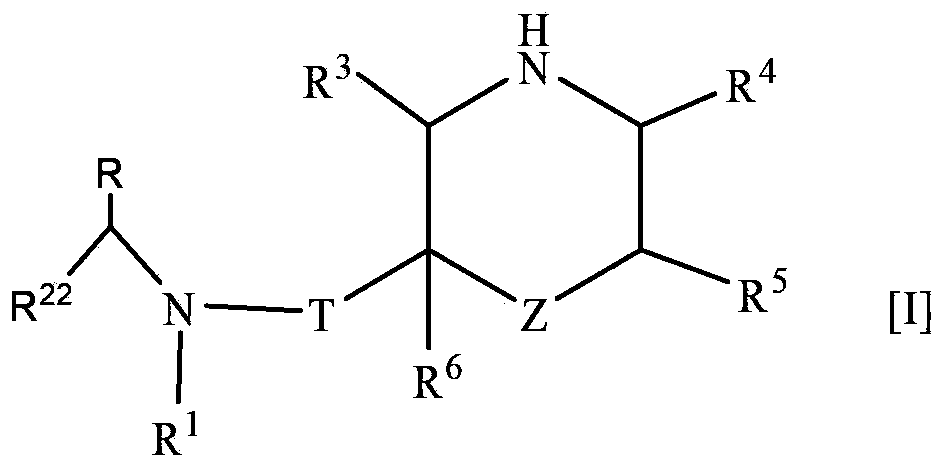
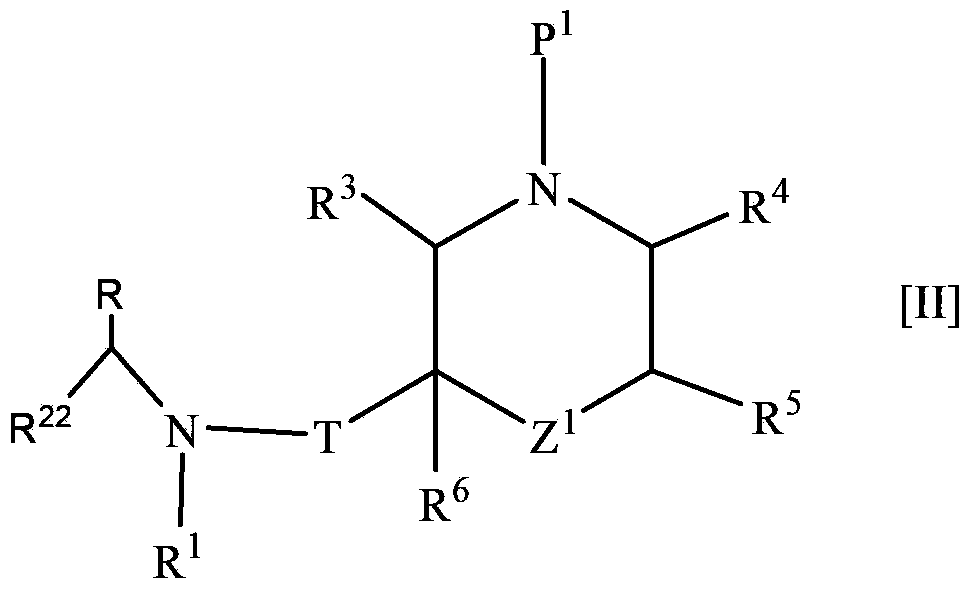
Nitrogen-containing saturated heterocyclic compound
A technology of compound and heterocyclic group, applied in the direction of medical preparations containing active ingredients, drug combination, organic chemistry, etc., can solve problems that do not involve details of morpholine compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment )
[0447] Specific examples (Examples) of synthesizing the objective compound [I] of the present invention by the methods exemplified above are shown below, but the present invention is not limited thereto.
Embodiment 1
[0450] (2R)-N-cyclopropyl-N-{1-[4-(3-methoxypropoxy)-1-benzofuran-6-yl]ethyl}morpholine-2-carboxamide[ Ex(1-1), Ex(1-2)]
[0451] [chemical formula 25]
[0452]
[0453] To (2R)-2-[(cyclopropyl{1-[4-(3-methoxypropoxy)-1-benzofuran-6-yl]ethyl}amino)carbonyl]morpholine-4 - To a solution of tert-butyl carboxylate (200mg) and 2,6-lutidine (0.142ml) in chloroform (4ml), add trimethylsilyl trifluoromethanesulfonate (0.180ml) under ice-cooling, in Stir at the same temperature for 30 minutes. Under ice-cooling, saturated aqueous sodium bicarbonate solution was added, followed by extraction with ethyl acetate. The organic phase was washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (eluent: chloroform / methanol / ammonia = 200 / 2 / 1) to give (2R)-N-cyclopropyl-N-{(1R)-1 as a colorless oil -[4-(3-Methoxypropoxy)-1-benzofuran-6-yl]ethyl}morpholine-2-carboxamide [Ex(1-1)] (1...
Embodiment 2
[0456] (2R)-N-{(1R)-1-[3-chloro-1-(3-methoxypropoxy)-1H-indolyl-6-yl]ethyl}-N-cyclopropyl Piperazine-2-carboxamide[Ex(2-1)]
[0457] [chemical formula 26]
[0458]
[0459] To (2R)-2-{[{(1R)-1-[3-chloro-1-(3-methoxypropyl)-1H-indolyl-6-yl]ethyl}(cyclopropyl )amino]carbonyl}piperazine-1,4-dicarboxylic acid tert-dibutyl ester (44.0mg) and 2,6-lutidine (0.050ml) in dichloromethane (1.0ml) solution, under ice cooling Trimethylsilyl trifluoromethanesulfonate (0.051 ml) was added, followed by stirring at the same temperature for 1 hour. Under ice-cooling, saturated aqueous sodium bicarbonate solution and methanol (2.0 ml) were added, extracted with chloroform, the organic phase was washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The resulting residue was purified by NH-silica gel chromatography (eluent: ethyl acetate→ethyl acetate / methanol=5 / 1) to give (2R)-N-{(1R)-1-[ 3-Chloro-1-(3-methoxypropyl)-1H-indolyl-6-yl]ethyl}-N-cy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com