Traditional Chinese veterinary medicinal preparation for treating swine enzootic pneumonia
A technology for swine asthma and veterinary drugs, applied in antiviral agents, medical preparations containing active ingredients, antibacterial drugs, etc., can solve the problems of loss of efficacy of antibiotics, increased drug dosage, and high recurrence rate, and avoid veterinary drug residues and The effect of social pollution, less dosage and less medicinal taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
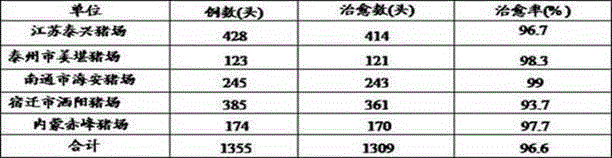
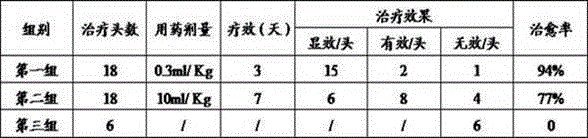
Image
Examples
Embodiment 1
[0045] Prescription: Toad 20g, the weight ratio is 1%, and the analgesic agent chlorobutanol is 0.5% by weight. The specific preparation process is as follows: Soak toads and hunchbacks in water for injection for 24-30 hours, decoct in water three times, 2 hours each time, combine and concentrate the filtrates from the three times until thick, add 95% ethanol to adjust the ethanol concentration to 60-70 degrees , fully stir and mix the precipitation, recover ethanol: after recovering the ethanol, add 95% ethanol to the liquid medicine to adjust the ethanol concentration to 75-85 degrees, stir and mix the precipitation, recover the ethanol, and then concentrate until there is no alcohol smell: add 2- 4 times of water for injection, refrigerated and precipitated, concentrated to 0.5g of crude drug per milliliter, to achieve the purpose of removing spirulina, protein, starch and resin in the liquid medicine. When boiling, add activated carbon to remove pyrogens, add additives to ...
Embodiment 2
[0047] Prescription: 40g toad, 60g hunchback, 1.5% solubilizer Tween-80 by weight, and 0.5% analgesic chlorobutanol to make a concentration of 0.5g crude drug per milliliter. The preparation method Ditto.
Embodiment 3
[0049] Prescription: 60g of toad, 90g of hunchback, 2% by weight of solubilizer Tween-80, and 0.5% by weight of analgesic chlorobutanol, made into a concentration of 0.5g of crude drug per milliliter, and its preparation method Ditto.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com