Preparation method of levo praziquantel as well as intermediate thereof
A technology of L-praziquantel and intermediates, which is applied in the field of intermediates for the preparation of L-praziquantel, can solve the problems of nitrile hydratase that is difficult to obtain and store, is not easy to use, and is cumbersome to operate, and achieves low production costs and improved yields , the effect of simple reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] step 1
[0048]
[0049] Dissolve compound HDP100-02 (90g, 361.45mmol) in isopropanol (1800mL), add (+)-α-methylbenzylamine (103g, 361.45mmol), heat to 60°C, the reaction solution is clear, and start to cool down slowly Crystallize overnight, filter with suction, rinse the filter cake with cold isopropanol (150 mL), and dry to obtain a white solid (79 g). Add isopropanol (1700mL) to the above white solid, heat and reflux until the solid is completely dissolved, slowly cool down and crystallize overnight, filter with suction, rinse the filter cake with cold isopropanol (100mL), and dry to obtain a white solid (55g ).
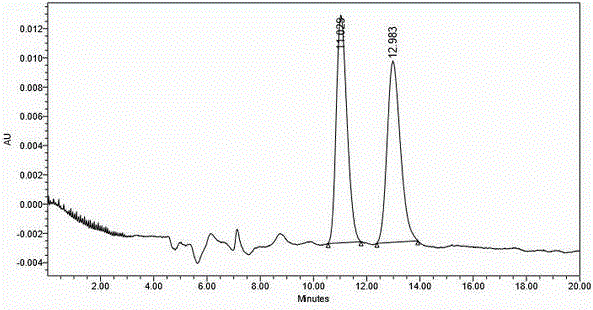
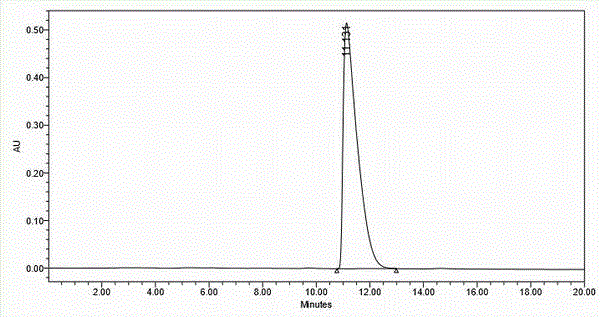
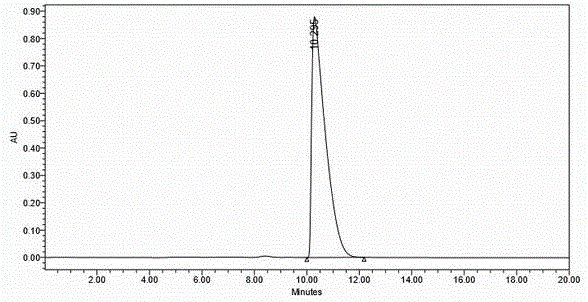
[0050] The white solid obtained by secondary crystallization was washed with saturated NaH 2 PO 4 The solution was freed, extracted with ethyl acetate, and the organic phase was washed with saturated NaCl solution (100 mL×1), dried over anhydrous sodium sulfate, and concentrated by suction to obtain an oily liquid (27 g), yield 30%.
[0051] The exp...
Embodiment 2
[0097] step 1
[0098]
[0099] Compound HDP100-02 (50g, 200.59mmol) was dissolved in isopropanol (1000mL), cinchonine (59g, 200.59mmol) was added, the reaction liquid was heated to 80°C, and the reaction liquid was clarified, then slowly cooled down to crystallize overnight, suction filtered, and the filter cake was Rinse with cold isopropanol (120 mL) and dry to give a white solid (72 g). Add isopropanol (1500mL) to the above white solid, heat and reflux until the solid is completely dissolved, slowly cool down and crystallize overnight, filter with suction, rinse the filter cake with cold isopropanol (150mL), and dry to obtain a white solid (50g ).
[0100] The white solid obtained by secondary crystallization was freed with 1N HCl aqueous solution, extracted with ethyl acetate, the organic phase was washed with saturated NaCl solution (100mL×1), dried over anhydrous sodium sulfate, and concentrated by suction to obtain an oily liquid (25g), yield 27.8 %.
[0101] The...
Embodiment 3
[0147] step 1
[0148]
[0149] Compound HDP100-02 (20g, 80.23mmol) was dissolved in isopropanol (400mL), and (R)-(+)-1-(1-naphthyl)ethylamine (11.06g, 64.18mmol) was added and heated to The reaction solution was clarified at 100°C, and the temperature was slowly lowered to crystallize overnight, filtered with suction, the filter cake was rinsed with cold isopropanol (100 mL), and dried to obtain a white solid (29 g). Add isopropanol (600mL) to the above white solid, heat and reflux until the solid is completely dissolved, slowly cool down and crystallize overnight, filter with suction, rinse the filter cake with cold isopropanol (150mL), and dry to obtain a white solid (20g ).
[0150] The white solid obtained by secondary crystallization was washed with 1NH 2 SO 4 The aqueous solution was freed, extracted with ethyl acetate, the organic phase was washed with saturated NaCl solution (100 mL×1), dried over anhydrous sodium sulfate, and concentrated by suction to obtain a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com