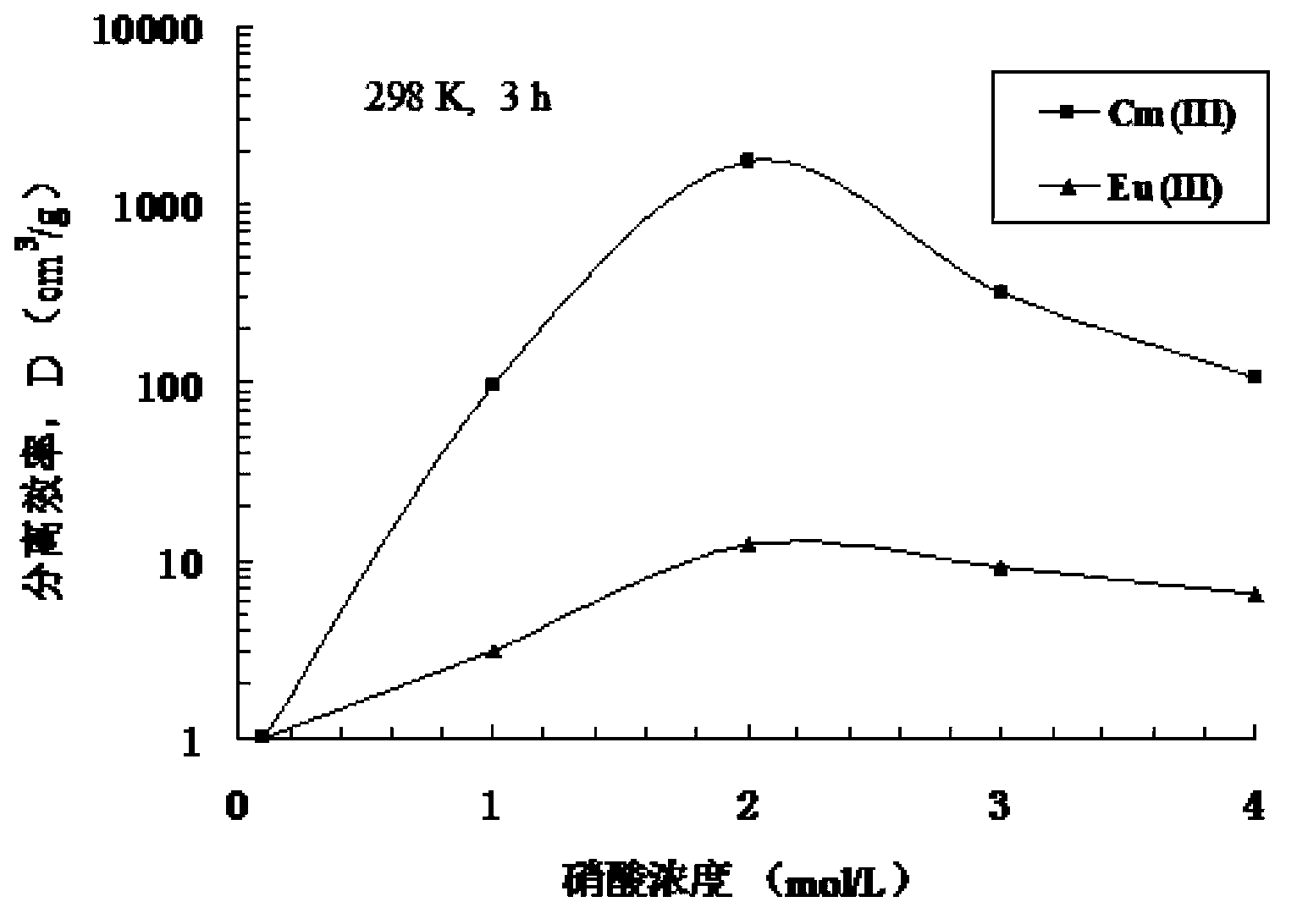
Dipyridine bridged bis-triazine compounds and preparation method thereof
A bipyridine bridge and bis-triazine technology is applied in the field of 2,2'-bipyridine bridge bis-triazine compounds, achieving the effects of mild conditions, high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
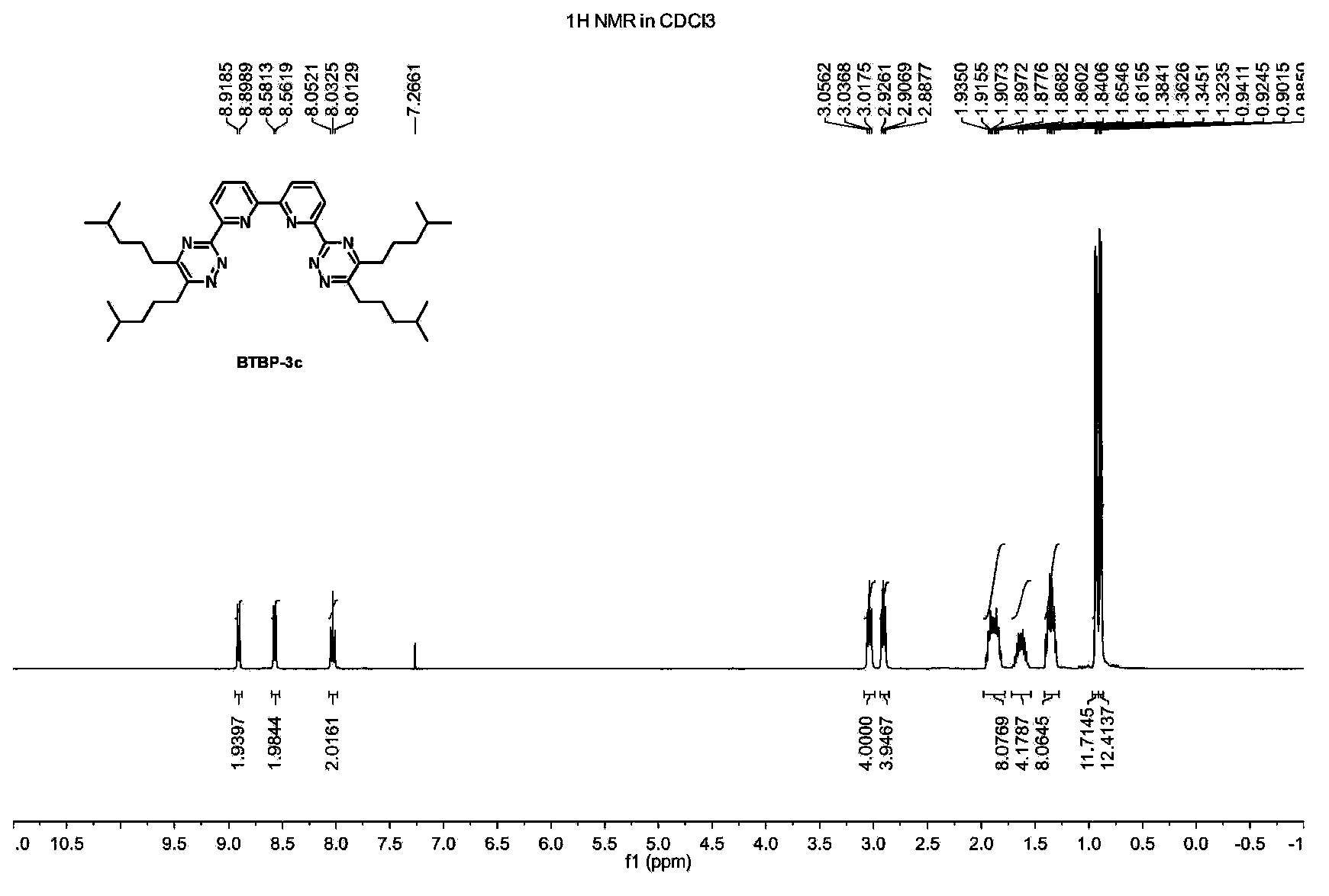
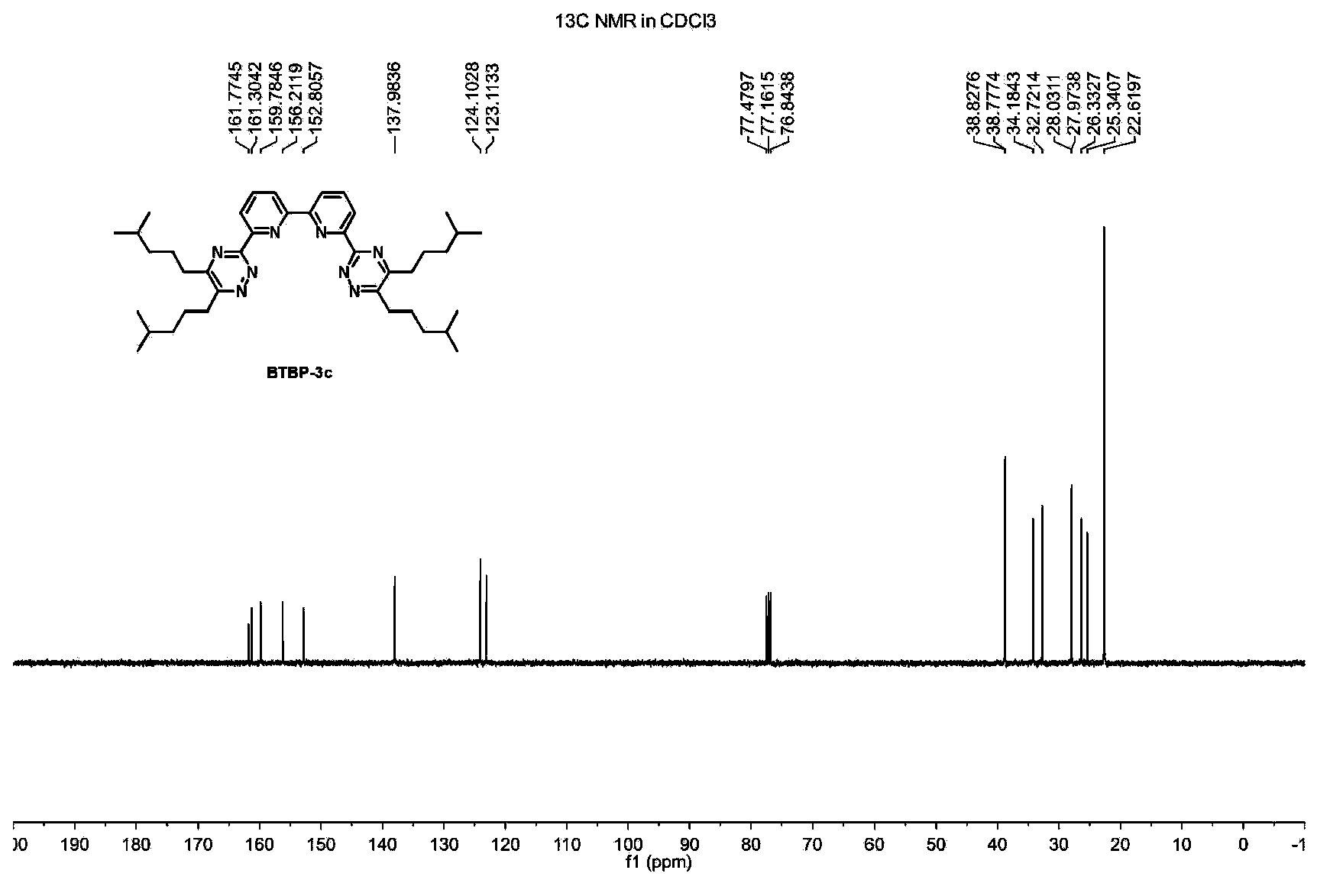
[0023] Example 1: Preparation of 6,6'-bis(5,6-diisobutyl-1,2,4-triazin-3-yl)-2,2'-bipyridine BTBP-3a
[0024] Add 2,2'-bipyridine-6,6'-diimide hydrazide (1) (0.54g, 2.0mmol), 2,7-dimethyl-4 , 5-octanedione (2a) (0.69g, 4.0mmol), 50mL tetrahydrofuran and 2mL triethylamine, magnetic stirring, heated to 65 ° C for 20 hours. The mixture was cooled to room temperature, filtered, and the filter cake was washed with 5 mL of tetrahydrofuran. The combined filtrates were removed under reduced pressure (20 mmHg) to remove volatile components. The crude product was separated by silica gel column chromatography, the eluent was dichloromethane, and the solvent was removed under reduced pressure (20mmHg) to obtain the target product BTBP-3a (0.65g, yield 60%), and its composition and molecular structure were obtained by nuclear magnetic resonance spectrum 1 H, 13 C{ 1 H} NMR and HRMS measurements were confirmed.
Embodiment 2
[0025] Example 2: Preparation of 6,6'-bis(5,6-diisobutyl-1,2,4-triazin-3-yl)-2,2'-bipyridine BTBP-3a
[0026] The reaction steps and operations were the same as in Example 1, except that the reaction time was 5 hours to obtain the target product BTBP-3a (0.47 g, yield 44%).
Embodiment 3
[0027] Example 3: Preparation of 6,6'-bis(5,6-diisobutyl-1,2,4-triazin-3-yl)-2,2'-bipyridine BTBP-3a
[0028] The reaction steps and operation are the same as in Example 1, except that the difference from Example 1 is that the reaction temperature is 20° C. to obtain the target product BTBP-3a (0.14 g, yield 13%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com