Method for preparing methanation catalyst by adopting urea combustion method
A methanation catalyst, catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., to achieve the effects of short process, increased specific surface area, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation method of the present embodiment comprises the following steps:
[0024] Step 1, take by weighing 340.3 grams of Al(NO 3 ) 3 9H 2 O, 14.6 g Ni(NO 3 ) 2 ·6H 2 O, 90.9 grams of urea, 27 grams of ethanol, put into a planetary ball mill and ball mill for 20 minutes, which is a fully mixed material for ball milling, wherein the ratio of the molar number of combustion agent urea to the sum of the molar numbers of aluminum nitrate and nickel nitrate is 3 :1.
[0025] Step 2. Transfer the material mixed uniformly by the ball mill to a crucible or a quartz boat, and directly put it into a muffle furnace with a preset temperature of 450°C for a combustion reaction, and keep it for 60 minutes, then cool naturally, which is the production process. 7.5%NiO / γ-Al 2 o 3 (NC) catalyst, (NC) means the catalyst prepared by the combustion method of aluminum source with aluminum nitrate as carrier.
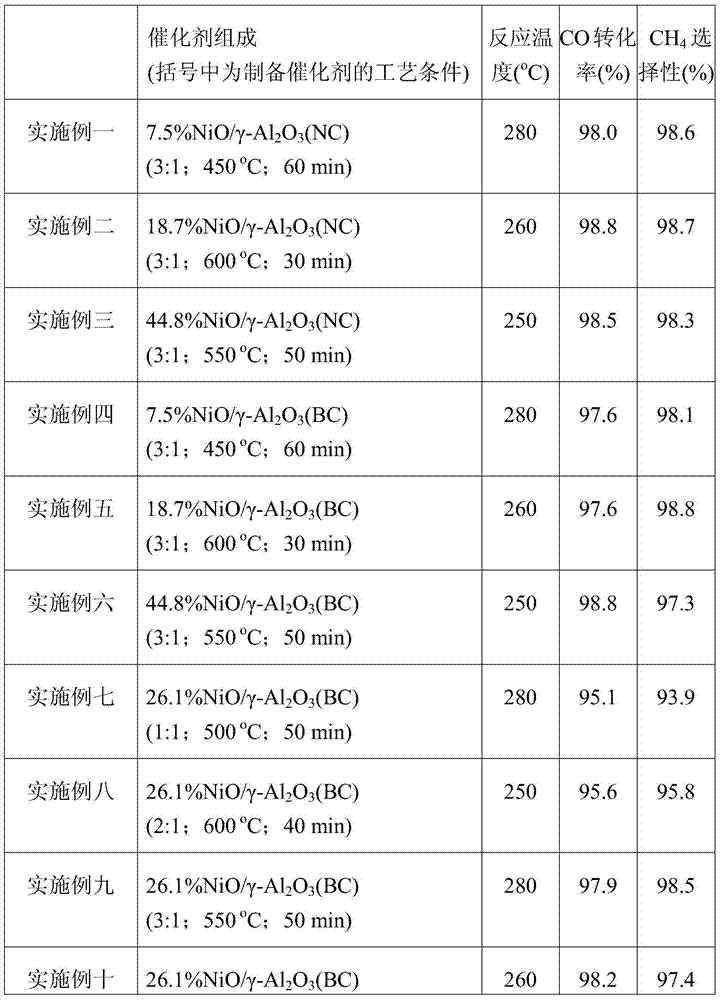
[0026] The composition and activity evaluation results of the cata...
Embodiment 2
[0028] The preparation method of the present embodiment comprises the following steps:
[0029] Step 1, take by weighing 299.1 grams of Al(NO 3 ) 3 9H 2 O, 36.4 g Ni(NO 3 ) 2 ·6H 2 O, 94.5 grams of urea, 52 grams of ethanol, put into a planetary ball mill and ball mill for 30 minutes, which is a fully mixed material for ball milling, wherein the ratio of the molar number of combustion agent urea to the sum of the molar numbers of aluminum nitrate and nickel nitrate is 3 :1.
[0030] Step 2. Transfer the material mixed uniformly by the ball mill to a crucible or a quartz boat, and directly put it into a muffle furnace with a preset temperature of 600°C for a combustion reaction, and keep it for 30 minutes, and then cool it naturally. 18.7%NiO / γ-Al 2 o 3 (NC) catalyst, (NC) means the catalyst prepared by the combustion method of aluminum source with aluminum nitrate as carrier.
[0031] The composition and activity evaluation results of the catalysts are shown in Table ...
Embodiment 3
[0033] The preparation method of the present embodiment comprises the following steps:
[0034] Step 1, take by weighing 203.1 grams of Al(NO 3 ) 3 9H 2 O, 87.2 g Ni(NO 3 ) 2 ·6H 2 O, 102.6 grams of urea, 74 grams of ethanol, put into a planetary ball mill and ball mill for 40 minutes, which is a fully mixed material for ball milling, wherein the ratio of the molar number of combustion agent urea to the sum of the molar numbers of aluminum nitrate and nickel nitrate is 3 :1.
[0035] Step 2. Transfer the material mixed uniformly by the ball mill to a crucible or a quartz boat, and directly put it into a muffle furnace with a preset temperature of 550°C for a combustion reaction, and keep it for 50 minutes, and then cool it naturally. 44.8%NiO / γ-Al 2 o 3 (NC) catalyst, (NC) means the catalyst prepared by the combustion method of aluminum source with aluminum nitrate as carrier.
[0036] The composition and activity evaluation results of the catalysts are shown in Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com