Synthetic method for Cilengitide by using thioesterase
A thioesterase and application technology, applied in the field of green chemical industry and enzyme catalysis, can solve the problems of high consumption, difficult to benefit the general public, high pollution of chemical total synthesis method, etc., to reduce synthesis cost, shorten catalysis time, and improve synthesis efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Construction of thioesterase prokaryotic expression vector
[0032] 1.1 Primer design
[0033] The following primers were designed according to the Microcystis thioesterase gene sequence BAA83994 published by GenBank. Upstream primer pET-28TE-F1: 5′-ATA GCT AGC ATC TAT GCT CAA GAG ATG AAT-3′, downstream primer pET-28TE-R1: 5′-TTC AAG CTT TTA CGA CTG TTT TGG GTT GAG-3′. The upstream and downstream primers introduce Nde I and Bam HI restriction sites (underlined), respectively, and the bases before the restriction sites are protected bases. Primers were synthesized by Shanghai Yingjun Biotechnology Co., Ltd.
[0034] 1.2 Cloning of thioesterase domain
[0035] Microcystis genomic DNA was extracted according to the instructions of the DNeasy plant Mini Kit50 reagent, and the amplified fragment was about 720bp. The DNA 2□l after rubber tapping and purified was used for the ligation reaction, and the system 5□l was used for transformation. Take 200□l of tra...
Embodiment 2
[0047] Embodiment 2: Thioesterase catalyzes the synthesis of Cilengitide product
[0048] The thioesterase protein prepared, expressed and purified in Example 1 is used as the source of the catalytic reaction enzyme (thioesterase can be artificially synthesized according to the sequence) to catalyze the synthesis of Cilengitide products. The catalytic synthesis steps are as follows: first, design and synthesize suitable for thioesterase catalysis Cilengtide's linear polypeptide substrate L-aspartyl-D-phenylalanyl-N-methyl-L-valyl-L-arginyl-glycyl; the substrate is artificially synthesized. Use phosphate buffer to dissolve and dilute it to a final concentration of 0.5 mM linear peptide, then add the TE protease prepared in Experimental Example 1, and make up to 500 μl with MOPS buffer, and finally make the concentration ratio of TE enzyme to linear substrate reach 1 :10. The mixture was incubated at a temperature of 24°C for 6 hours and immediately placed at -70°C to stop the ...
Embodiment 3
[0053] Example 3: Comparison of the efficiency of TE enzyme cyclization Cilengitide synthesis with traditional chemical methods
[0054] 3.1TE enzyme catalyzes the synthesis of Cilengitide
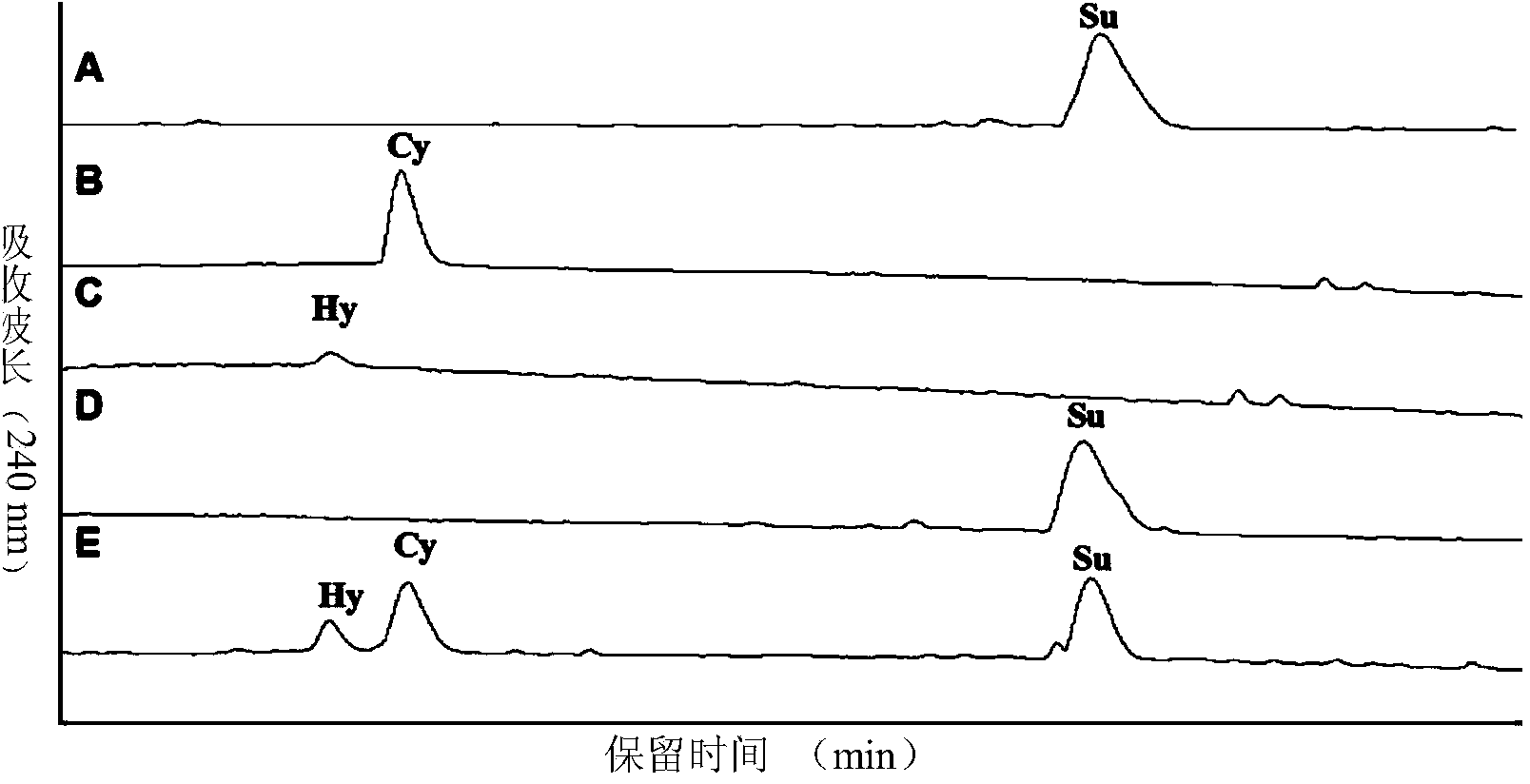
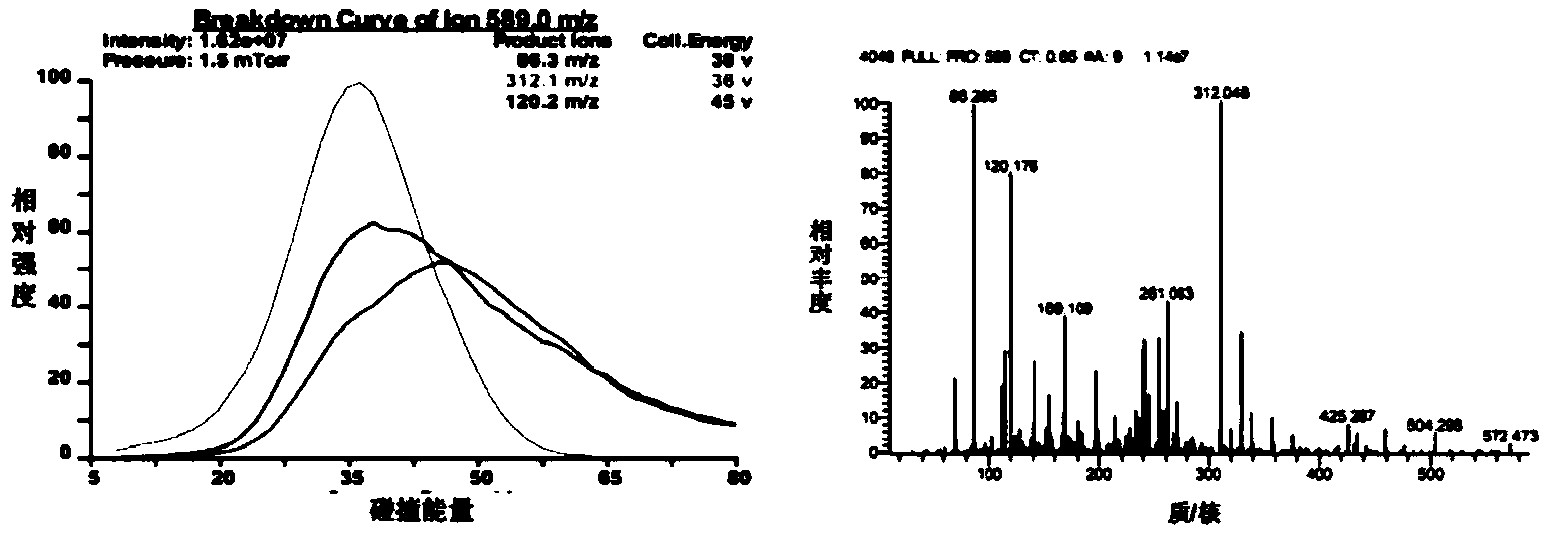
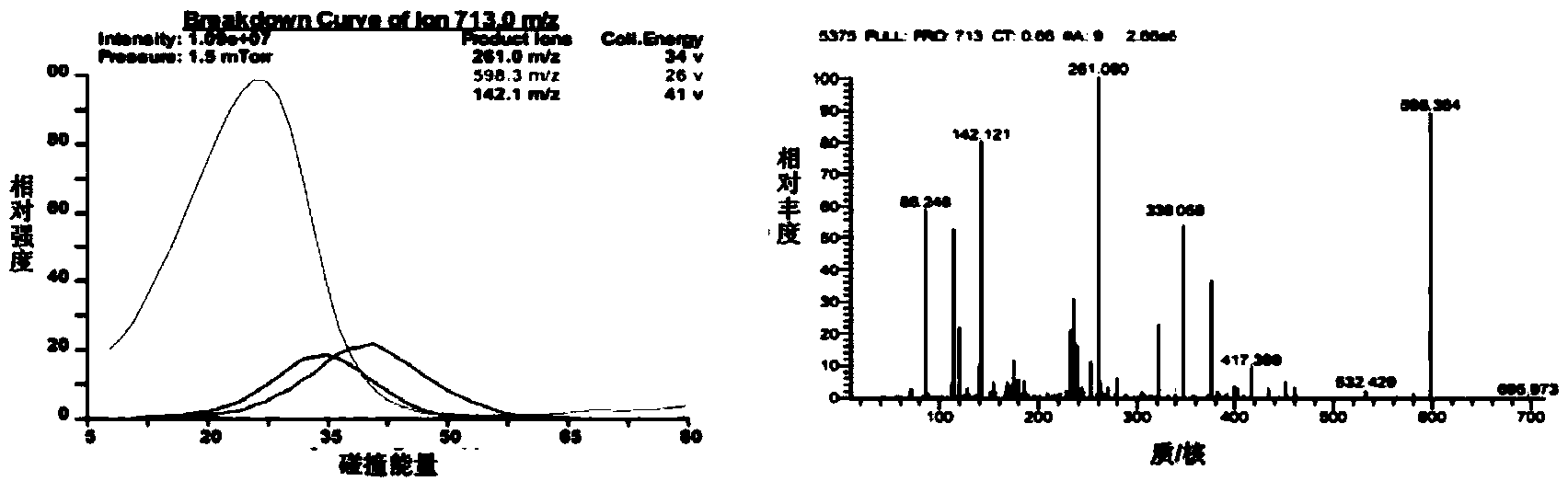
[0055] In the linear peptide Cilengitide 0.5g Tirs buffer: add supersaturated TE enzyme, and finally make up to 1000mL with Tris buffer to form the final TE catalytic system. The catalytic system was incubated at 24°C for 6h, and immediately placed at -70°C to make After the enzyme-catalyzed reaction is stopped, the reaction solution should be concentrated and dried under reduced pressure to obtain the crude cyclic peptide. The retention time and relative molecular weight of the cyclic peptide target main peak on HPLC determined according to the retention time of Cilengitide on HPLC and MS-MS analysis. The crude cyclic peptide was purified by preparative HPLC, the amount of the purified cyclic peptide was calculated, and the yield of Cilengitide catalyzed by the TE enzyme was calculated b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com