Method for purifying N-(2',6'-xylyl)-2-piperidinecarboxamide type local anesthetic
A technology of piperidine carboxamide and purification method, which is applied in the field of purification of N--2-piperidine carboxamide local anesthetics, can solve the problems of insignificant impurity removal effect, large amount of organic solvent, low yield, etc., and achieve dissolution Small amount, less time-consuming, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
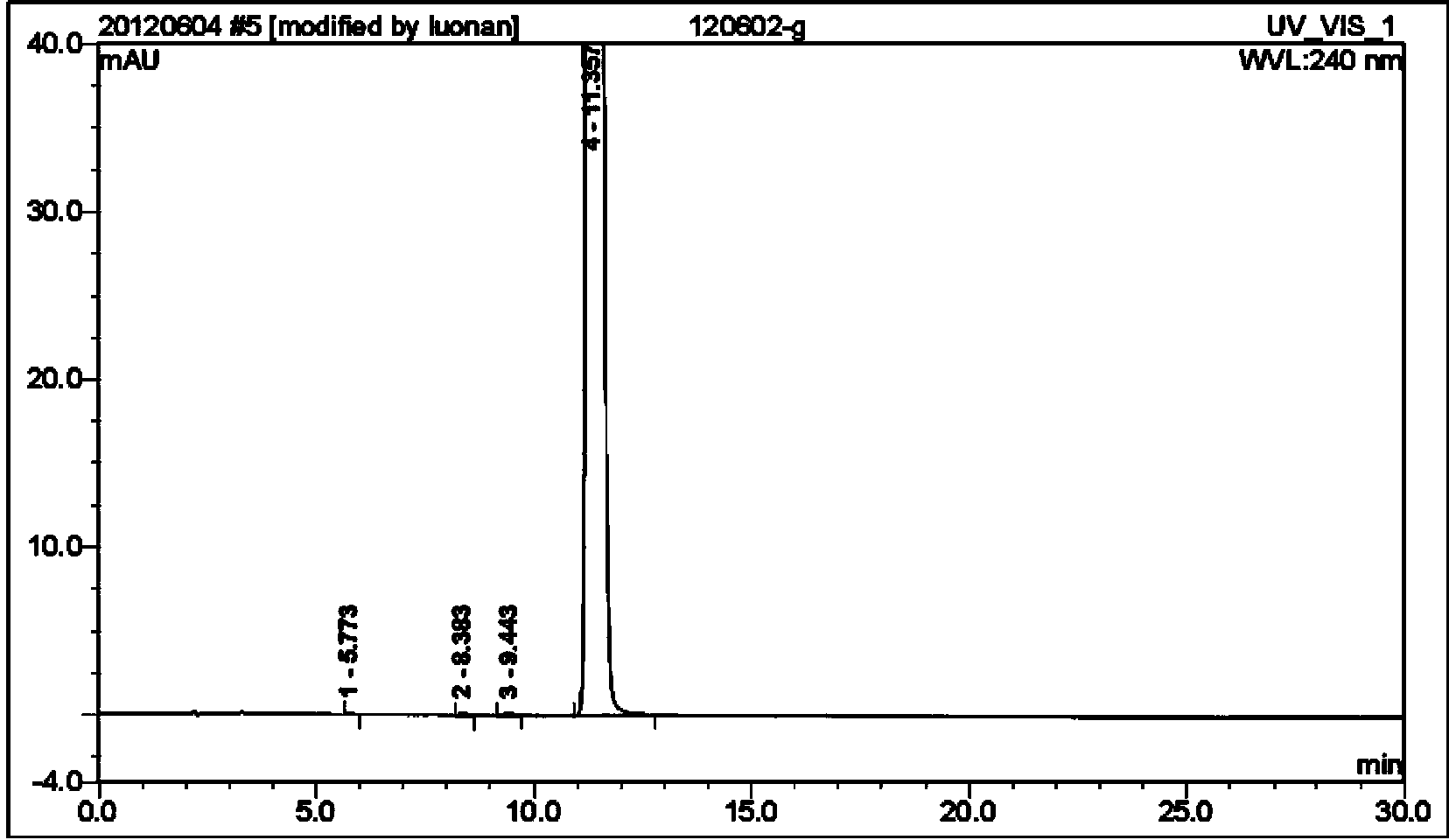
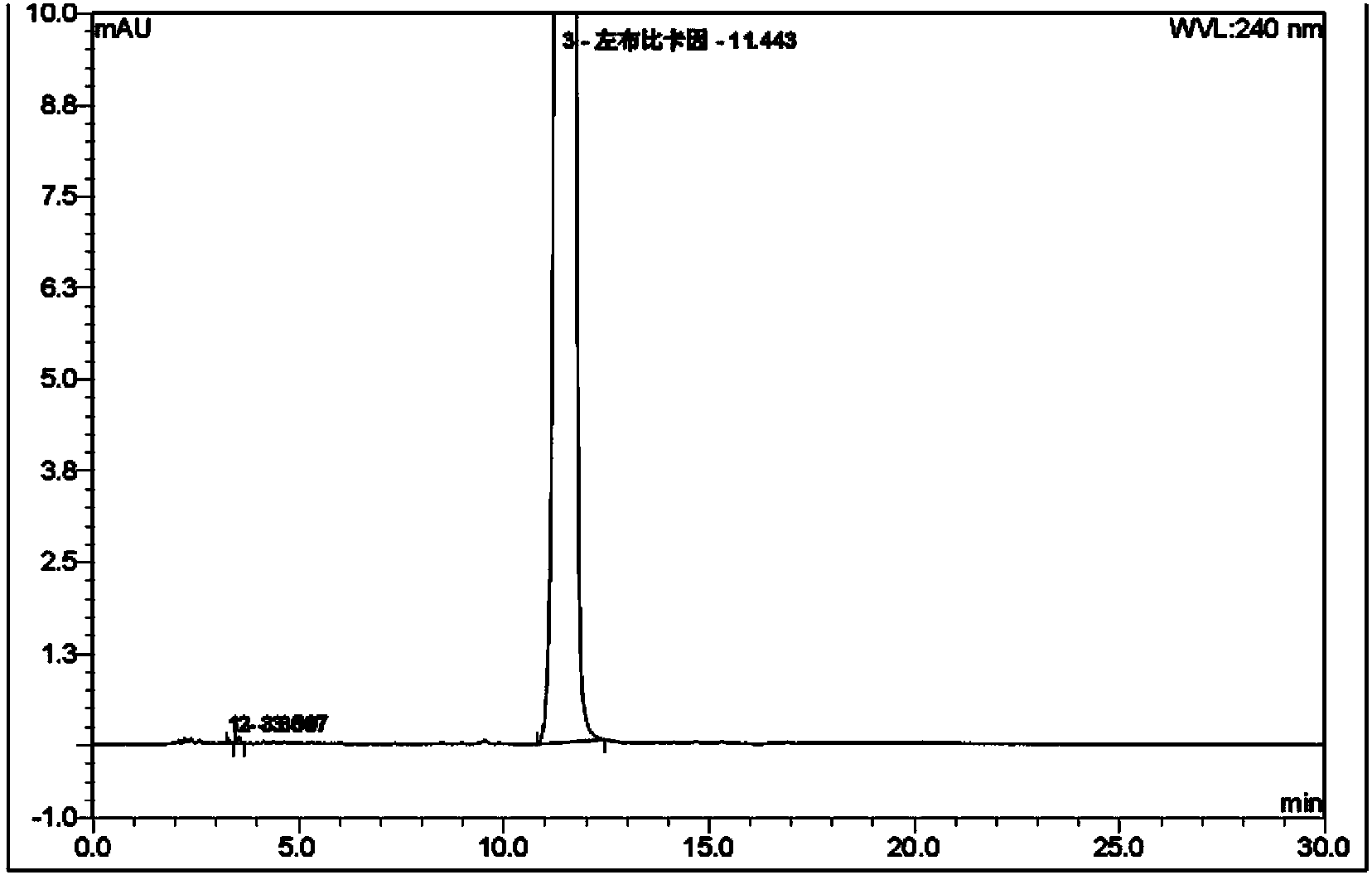
[0051] 5g levobupivacaine crude product (HPLC purity 96.3%) was added in the mixed solvent of 2ml acetone and 10ml water, polished 1 hour with rubber mill, filtered, dried to obtain 4.8g bupivacaine refined product (yield is 96 %, HPLC purity is 99.21%), and its particle size coefficient is less than 20 μm. Refined product is added in the mixed solvent of 5ml acetone and 5ml ethanol, add hydrochloric acid to form a salt when grinding with rubber mill, polish 1.5 hours, filter, dry to get 3.9g bupivacaine hydrochloride (yield is 72%, HPLC purity is 99.97%), and its particle size coefficient is less than 2 μm.
Embodiment 2
[0053] 5g levobupivacaine crude product (HPLC purity 96.3%) was added in the mixed solvent of 2ml methyl ethyl ketone and 15ml water, polished 2 hours with rubber mill, filtered, dried to obtain 4.6g bupivacaine refined product ( The yield is 92%, the HPLC purity is 99.15%), and its particle size coefficient is less than 15 μ m. Refined product is added in the mixed solvent of 5ml methyl ethyl ketone and 10ml methyl alcohol, adds hydrochloric acid to form salt when grinding with rubber mill, grinds 1.5 hours, filters and dries to get 3.7g bupivacaine hydrochloride (yield is 71% , HPLC purity is 99.95%), and its particle size coefficient is less than 1 μm.
Embodiment 3
[0055] 5g levobupivacaine crude product (HPLC purity 96.3%) was added in the mixed solvent of 2mlDMF and 15ml water, polished 1 hour with rubber mill, filtered, dried to obtain 4.5g bupivacaine refined product (yield is 90% , HPLC purity is 99.36%), and its particle size coefficient is less than 20 μm. Refined product is added in the mixed solvent of 4ml acetone and 8ml ethanol, add hydrochloric acid to form a salt when grinding with rubber mill, polish 1.5 hours, filter, dry to get 3.6g bupivacaine hydrochloride (yield is 71%, HPLC purity is 99.98%), and its particle size coefficient is less than 2 μm.
[0056] Add 54g (S)-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide, 42g potassium carbonate, 150ml N,N-dimethylformamide, 26ml bromopropane into the three-necked flask, The temperature was raised to 83°C, and the reaction was stirred. After the reaction was completed, the reaction mixture was poured into 600 ml of ice water and stirred. After suction filtration, the filter c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com