A compound long-acting in situ gel injection for treating chronic hepatitis B and its preparation method
A gel injection, chronic hepatitis B technology, applied in the field of medicine, can solve the problems of patients forgetting to take medicine, affecting curative effect, and long treatment cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
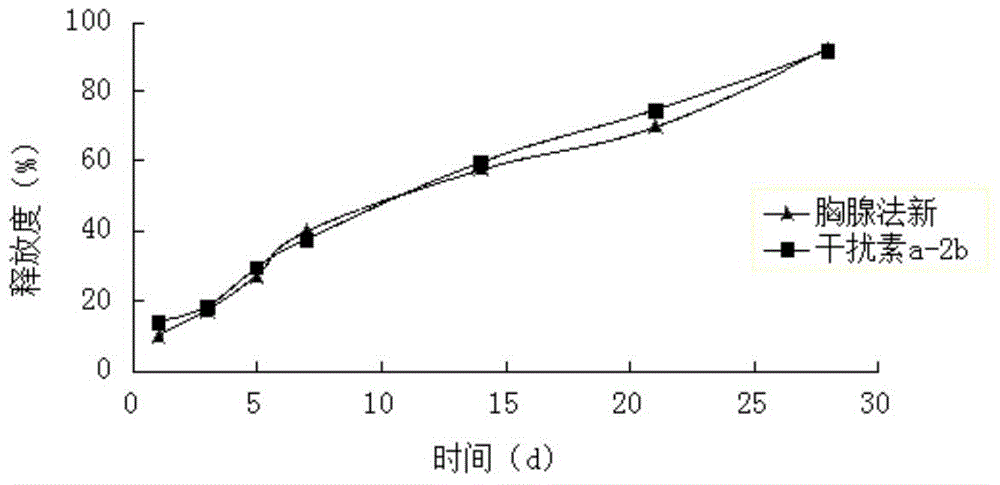
[0064] Weigh 15 mg of interferon a-2b and dissolve it in sterile water for injection, add 35 mg of zinc hydroxide that has been micronized and sterilized, and vortex and mix for 10 minutes to form 1 g of zinc salt interferon a-2b solution.
[0065] Weigh 2.6g PLGA (weight average molecular weight 10000, intrinsic viscosity 0.10dl / g-0.20dl / g, LA:GA=75:25), 0.9g poloxamer 407 and 18g NMP, mix well, and prepare polymer solution , then add 0.30g of Thymofaxin raw material, ultrasonically dissolve, after complete dissolution, add 1g of zinc salt interferon a-2b solution, heat at constant temperature and stir at high speed (temperature 30°C, 10000rpm) for 5min, to form a drug-loaded sol system containing particles , after electron beam sterilization, the compound long-acting in situ gel injection is obtained. The drug loading amount of interferon was detected by HPLC to be 0.37%, and the encapsulation efficiency was 95%. The drug loading of thymus method was 7.18%, and the encapsul...
Embodiment 2
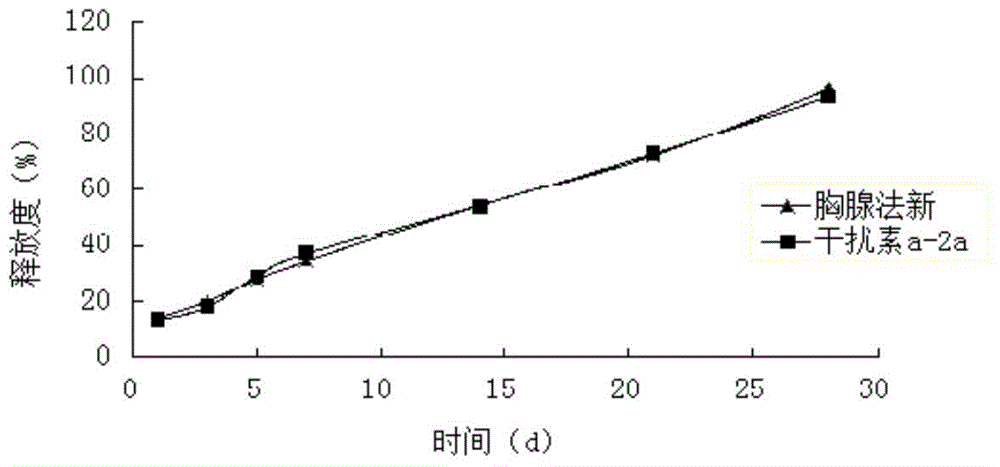
[0069] Weigh 20 mg of interferon a-2a and dissolve it in sterile water for injection, add 55 mg of zinc chloride after micronization and sterilization, and vortex and mix for 10 minutes to form 1 g of zinc salt interferon a-2a solution.
[0070] Weigh 4.0g PLGA (weight-average molecular weight 15000, intrinsic viscosity 0.10dl / g-0.25dl / g, LA:GA=65:35), 2.0g methylcellulose and 45g ethyl lactate, mix well, and prepare a polymer Then add 0.80g of Thymusfaxin raw material drug, ultrasonically dissolve, add 1g of zinc salt interferon a-2a solution after complete dissolution, heat at constant temperature and stir at high speed (temperature 30°C, 10000rpm) for 7min to form drug-loaded particles The sol system is sterilized by electron beams to obtain the compound long-acting in-situ gel injection. The drug loading amount of interferon was detected by HPLC to be 0.33%, and the encapsulation efficiency was 97%. The drug loading capacity of thymus method was 12.53%, and the encapsulat...
Embodiment 3
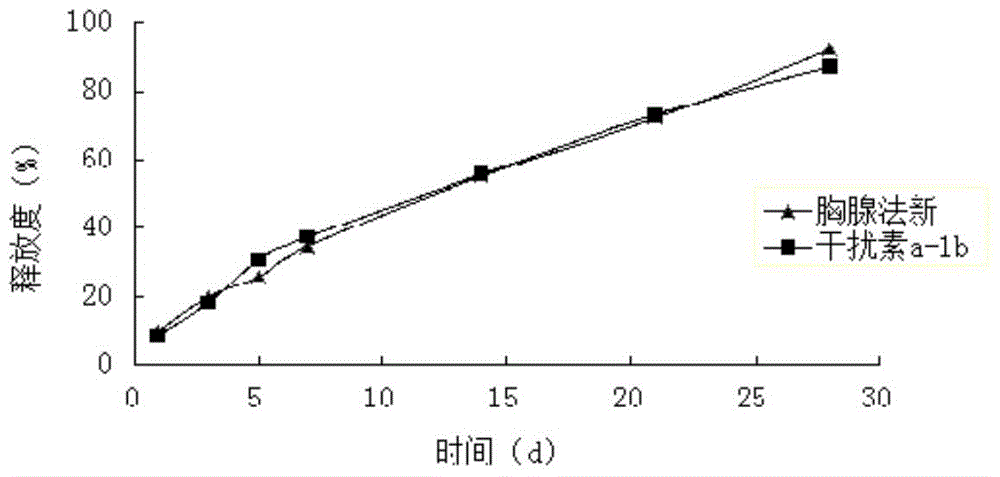
[0074] Weigh 25 mg of interferon a-1b and dissolve it in sterile water for injection, add 55 mg of magnesium hydroxide that has been micronized and sterilized, and vortex and mix for 10 minutes to form a solution of 1 g of magnesium salt interferon a-1b.
[0075] Weigh 3.75g PLA (weight-average molecular weight 15000, intrinsic viscosity 0.10dl / g-0.25dl / g), 3.75g Carbomer 934 and 35g acetone, mix well, prepare polymer solution, and then add thymus method new API 0.75g, ultrasonically dissolved, after complete dissolution, add 1g of magnesium salt interferon a-1b solution, heat at constant temperature and high-speed stirring (temperature 30°C, 10000rpm) for 5min, to form a drug-loaded sol system containing particles, sterilized by electron beams, The compound long-acting in situ gel injection is obtained. The drug loading amount of interferon was detected by HPLC to be 0.28%, and the encapsulation efficiency was 98%. The drug loading of thymus method was 12.44%, and the encaps...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com