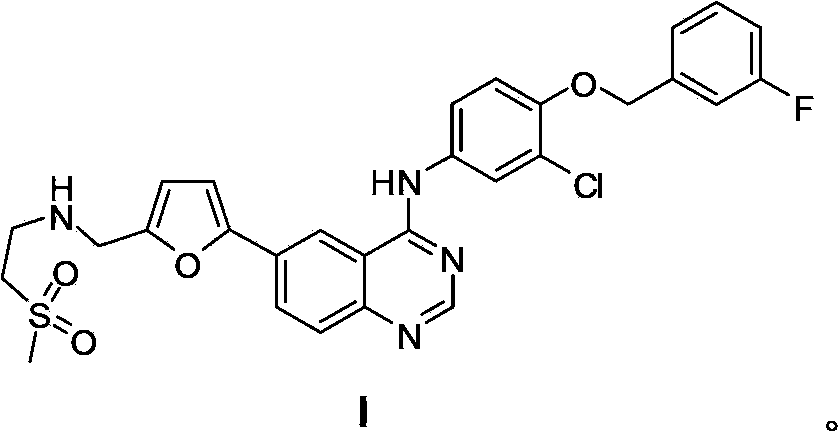
New preparation method of lapatinib
A compound, optionally a technology, applied in the field of medicine to achieve the effect of high yield, high purity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Weigh 100g of compound 1 in a 2L three-neck flask, stir mechanically, add 1000mL acetone and 76gK 2 CO 3 , heated to 20°C. After reacting for half an hour, 104 g of compound 2 was slowly added dropwise. After the addition, the reaction was carried out at 60°C for 4 hours. After the reaction, cool to room temperature, add water into the reaction bottle, stir, filter with suction, and wash the solid with ethanol to obtain compound 3, with a mass of 132 g and a yield of 94%.
Embodiment 2
[0051] Weigh 100g of compound 1 in a 2L three-neck flask, stir mechanically, add 1000mL CH 3 CN and 76g K 2 CO 3 , react at room temperature, react for half an hour and then slowly add 104g of compound 2 dropwise, after the addition, heat to reflux for 4 hours. After the reaction, cool to room temperature, add a large amount of water into the reaction bottle, stir, filter with suction, and wash the solid with a small amount of ethanol to obtain compound 3, with a mass of 134 g and a yield of 95%. 1 H-NMR (CDCl 3 )δ: 5.25(s,2H),7.00~7.07(m,2H),7.17~7.23(m,1H),7.26(s,1H),7.36~7.41(m,1H),8.11~8.14(m, 1H),8.29(d,J=3.2HZ,1H). 13 C-NMR (CDCl 3 )δ: 70.45(d, J=6.8HZ), 112.43, 113.85(d, J=88.8HZ), 115.38(d, J=83.6HZ), 122.41(d, J=12HZ), 123.83(d, J= 40HZ),126.16,130.47(d,J=32.8HZ),137.48(d,J=29.2HZ),141.53,158.89,161.82,164.27.MS(m / z):280.0[M-H] - .
Embodiment 3
[0053] Put 132g of compound 3 into a 2L three-neck flask, add 50mL of water and 1000mL of glacial acetic acid, stir, and heat to 50°C. After compound 3 is dissolved, slowly add 131g of iron powder in batches. After the addition, keep at 50°C for 30min to reduce When the temperature reached 25°C, the raw material disappeared, and the reaction was stopped. Add EA and water, separate the layers, and extract. The organic phase was washed three times with water, washed three times with saturated brine, dried, concentrated, and then added 6N~12N concentrated hydrochloric acid to make the product salted out, filtered, and then the solid and aqueous phase were adjusted to a basic pH, extracted with ethyl acetate, and dried. Spin-dried to obtain compound 4 with a mass of 82 g and a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com