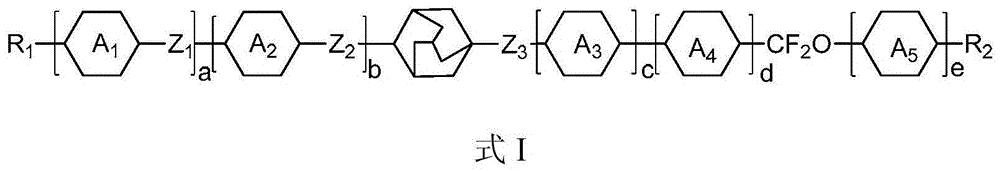
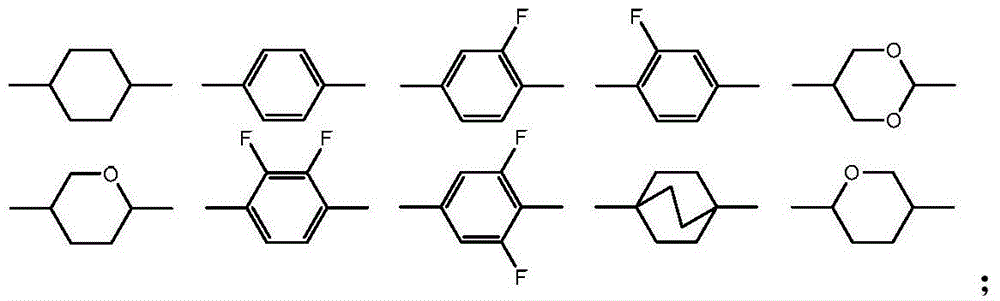
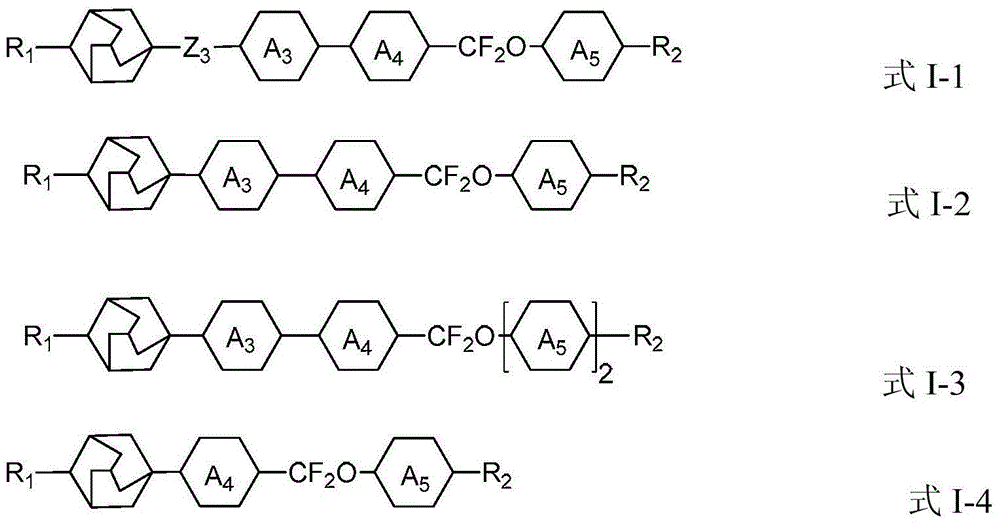
Adamantane derivatives and their preparation methods and applications
A kind of compound, straight-chain alkyl technology, applied in the field of adamantane derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1 Synthesis of Compound I-13
[0114]
[0115] Step 1 Synthesis of I-13-a
[0116] Add 29.1g (0.1mol) 4-(1-adamantane)p-bromobenzene (reactant), 80ml of tetrahydrofuran (solvent) into the reaction flask, protect by nitrogen, cool to -60℃, add 0.1mol n-butyl dropwise The petroleum ether (solvent) solution of lithium (reactant) was added dropwise within 1 hour, and the reaction was stirred at -50°C for 30 minutes. Then the temperature was lowered to -60°C, and 13g (0.13mol) of trimethyl borate (reactant) in 70ml of tetrahydrofuran (solvent) solution was added dropwise within 1 hour. After the addition, the reaction was continued with temperature control and stirring for 1 hour, and the temperature was raised to room temperature. Add 0.2mol hydrochloric acid and stir for 1 hour. After washing with water, extract with 50ml of ethyl acetate (solvent) and separate the liquids. Wash the organic phase with water until it is neutral. After evaporating the solvent, 20.5g of c...
Embodiment 2
[0128] Example 2 Synthesis of Compound I-14
[0129]
[0130] Add 28.2g (0.11mol) of I-13-1 (reactant) in Example 1, 48.3g (0.1mol) gP into the reaction flask 3 (Reactant), 0.3g of tetrakistriphenylphosphine palladium (catalyst), 15g of sodium carbonate (catalyst), 100ml of toluene (solvent), 100ml of water, 100ml of ethanol (solvent), heat and reflux for 4 hours, add 100ml of water, divide The organic phase was evaporated to dryness, and the product was obtained by column chromatography and recrystallization. The yield of 43 g was 70%.
[0131] The test results are as follows:
[0132] GC: 99.9%
[0133] MP: 178°C
[0134] CP: 232°C
[0135] MS: 614 (3.5) 467 (100) 346 (16.7) 359 (8.0)
[0136] 1H-NMR: δ(ppm:) 1.72(t,6H), 1.87(m,3H), 1.99(d,6H), 6.89(m,2H), 7.02(d,1H), 7.22(d,2H) , 7.38 (s, 4H) 7.58 (d, 1H), 7.83 (m, 1H)
[0137] It can be seen from the above that the product has a correct structure and belongs to the target product of formula I.
[0138] The liquid crystal performance t...
Embodiment 3
[0150] Example 3. Preparation of liquid crystal composition a
[0151] Mix the compounds according to the following mass percentages to obtain liquid crystal composition a:
[0152]
[0153]
[0154] The performance test results of the liquid crystal composition are as follows:
[0155] Cp=98℃
[0156] △n=0.105
[0157] △ε=7.5
[0158] It can be seen from the above that the liquid crystal composition has a wide temperature range of the liquid crystal phase, low viscosity, suitable refractive index anisotropy and low starting voltage, and has important application value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com