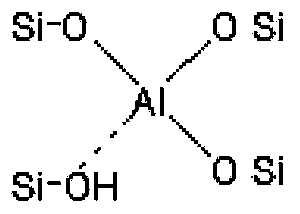
Preparation method for immobilized Lewis acid
A technology of concentrated nitric acid and molecular sieves, which is applied in the preparation of carboxylic acid esters, organic compounds, sugar derivatives, etc., can solve the problems of high price, long production cycle, and inapplicability to large-scale preparation, and achieve wide applicability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of tin-containing molecular sieve (Sn-Beta): Mix 1 part of hydrogen-type β molecular sieve (H-β) with 20 parts of 13M nitric acid solution and heat to 100°C for 20 hours. Filtrate, wash with water until neutral, dry to remove water, and obtain dealuminated molecular sieve (deAlBeta). Then add to dissolve 10 parts by weight of anhydrous SnCl 4 In 100 parts of benzene, stirred at 25°C for 24h, filtered, washed with 1000mL benzene to remove free SnCl 4 , 550 ° C air activation 3h, after cooling to get Sn-Beta.
Embodiment 2
[0025] Synthesis of Ti-containing molecular sieve (Ti-Beta): Mix 1 part of hydrogen-type β molecular sieve (H-β) with 50 parts of 13M nitric acid solution and heat to 50°C for 48 hours. Filtrate, wash with water until neutral, dry to remove water, and obtain dealuminated molecular sieve (deAlBeta). Then add to dissolve 0.1 weight part of anhydrous TiCl 4 In 5 parts of chloroform, stirred at 10°C for 48h, filtered, washed with 500 parts of toluene to remove free TiCl 4 , Activated by air at 400°C for 20h, and obtained Ti-Beta after cooling.
Embodiment 3
[0027] Synthesis of In-containing molecular sieve (In-Beta): Mix 1 part of hydrogen-type β molecular sieve (H-β) with 50 parts of 8M nitric acid solution and heat to 150°C for 1 hour. Filtrate, wash with water until neutral, dry to remove water, and obtain dealuminated molecular sieve (deAlBeta). Then add to the solution with 10 parts by weight of anhydrous InCl 3 In 10 parts of methanol, stirred at 65°C for 48h, filtered, washed with 500 parts of chloroform to remove free InCl 3 , 800 ° C air activation 1h, after cooling In-Beta.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com