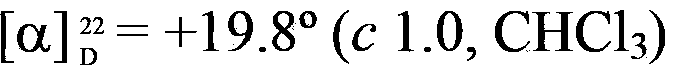Rhodococcus baikonurensis and application thereof in preparing optically pure (R)-6-hydroxy-8-chlorocaprylate and other optically active chiral alcohol
An optically active, ethyl chlorooctanoate technology for use in microorganism-based methods, bacteria, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The screening of embodiment 1 bacterial strain
[0030] Prepare enriched medium with the following components: (NH 4 ) 2 SO 4 1.0g / L, KH 2 PO 4 3.0g / L, K 2 HPO 4 6.0g / L, MgSO 4 0.5g / L, CaCl 2 0.05g / L; another rich medium is prepared, the composition is as follows: glucose 15g / L, yeast extract 5g / L, peptone 5g / L, KH 2 PO 4 1.0g / L, K 2 HPO 4 1.0g / L, MgSO 4 0.5g / L, pH7.0. Put 50mL enrichment medium and 2mM substrate in a 250mL Erlenmeyer flask as a carbon source, add soil samples, and culture at 30°C and 180rpm for 1-6 days. Afterwards, the culture solution is spread on a rich medium plate, cultured at 30°C for 1-3 days, and the grown single colonies are separated and purified by streaking on the plate. The substrate is ethyl 6-carbonyl-8-chlorooctanoate.
[0031] Inoculate the obtained single colony into the rich liquid medium, culture at 30°C, 180rpm for 2 days, take a certain amount of wet cells (0.1 ~ 0.5g) and suspend in 1mL of phosphate buffer (PBS, 50...
Embodiment 2
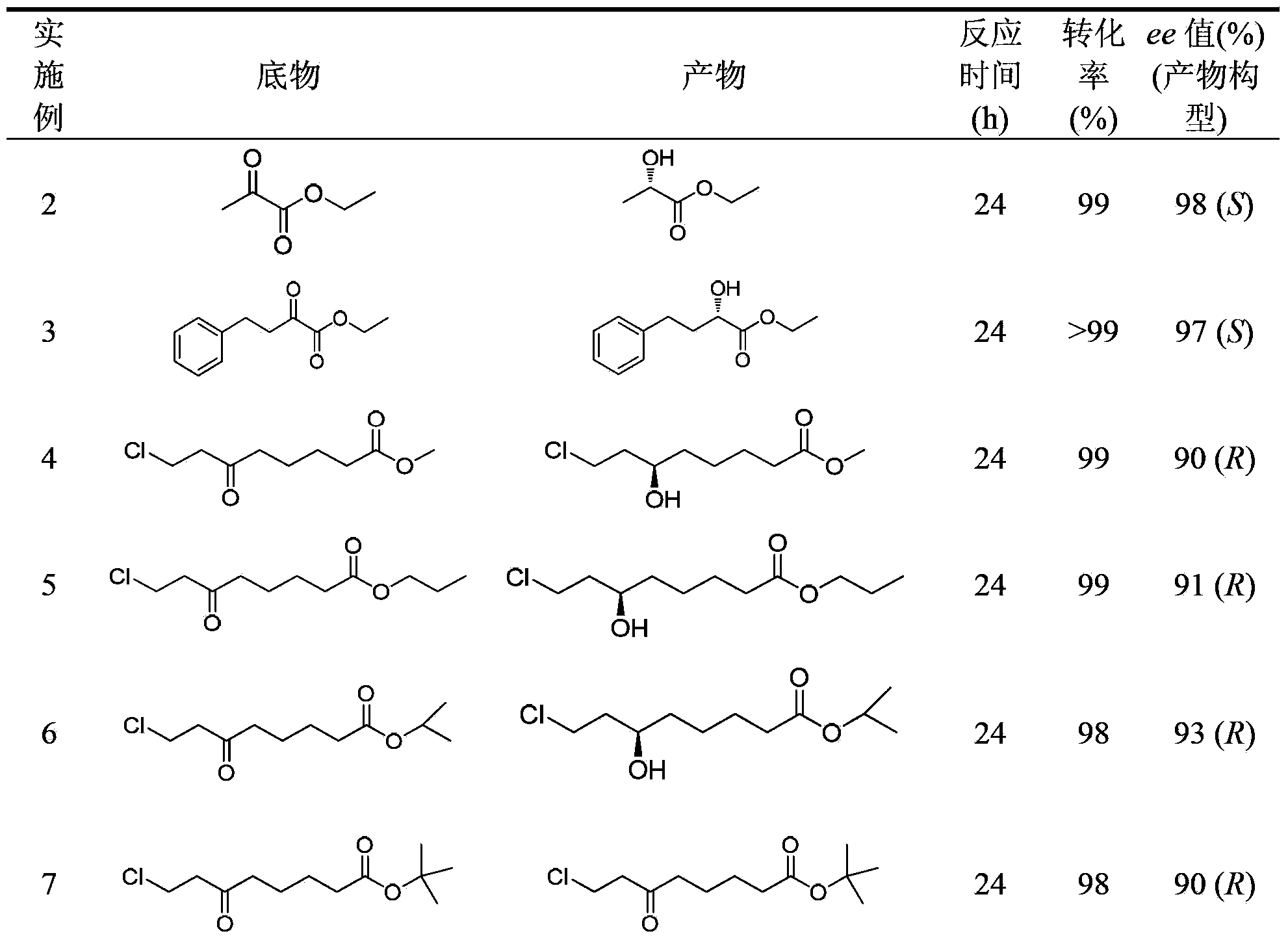
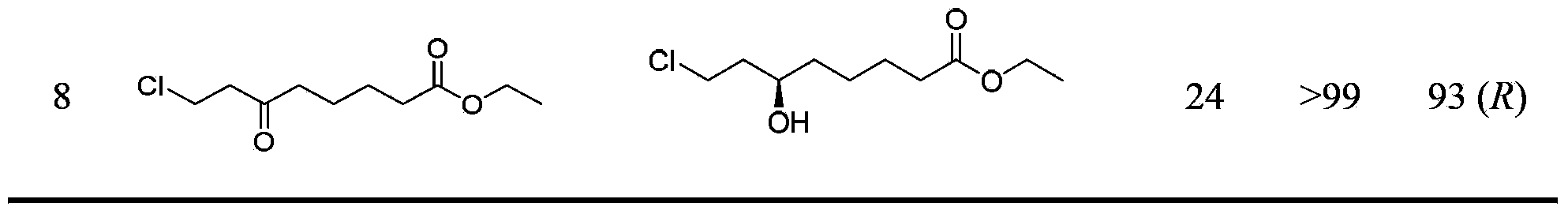
[0036] Example 2-8 Resting cells catalyze the asymmetric reduction of carbonyl compounds
[0037] Add 0.01g of Rhodococcus baikonurensis ECU1014 resting cells and 2U of glucose dehydrogenase crude enzyme solution to 1mL sodium phosphate buffer (100mmol / L, pH6.0) 641), respectively adding the latent chiral carbonyl compound (Example 2-8) with a final concentration of 10mmol / L, and the NAD with a final concentration of 0.5mmol / L + And 3g / L of glucose. The reaction was shaken at 1100 rpm for 24 h at 30°C. After the reaction was completed, extract with an equal volume of ethyl acetate, extract twice, combine the extracts, add anhydrous sodium sulfate to dry overnight, and then analyze and measure the conversion rate of the substrate and the ee value of the reduced product. The results are shown in Table 1.
[0038] The specific analysis conditions for the ee value of the product are as follows:
[0039] Use a gas chromatograph for analysis, the chromatographic column is a chira...
Embodiment 3
[0041] Embodiment 3: column temperature 160 ℃;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com