Method for acquiring goose OAZ1 gene full length CDS sequence and detecting expression rule thereof quickly
A genetic and rapid technology, applied in the field of rapid acquisition of goose OAZ1 gene full-length CDS sequence and rapid detection of its expression law, can solve problems such as errors, inability to understand accurate changes in the quantitative process, and inability to accurately detect the expression of target genes. Achieve the effects of improving accuracy, avoiding unsuccessful splicing, and saving test costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0021] A method for rapidly obtaining the full-length CDS sequence of goose OAZ1 gene and rapidly detecting its expression law comprises the following steps:
[0022] 1. Take the total RNA in goose ovary tissue and reverse transcribe it into cDNA;
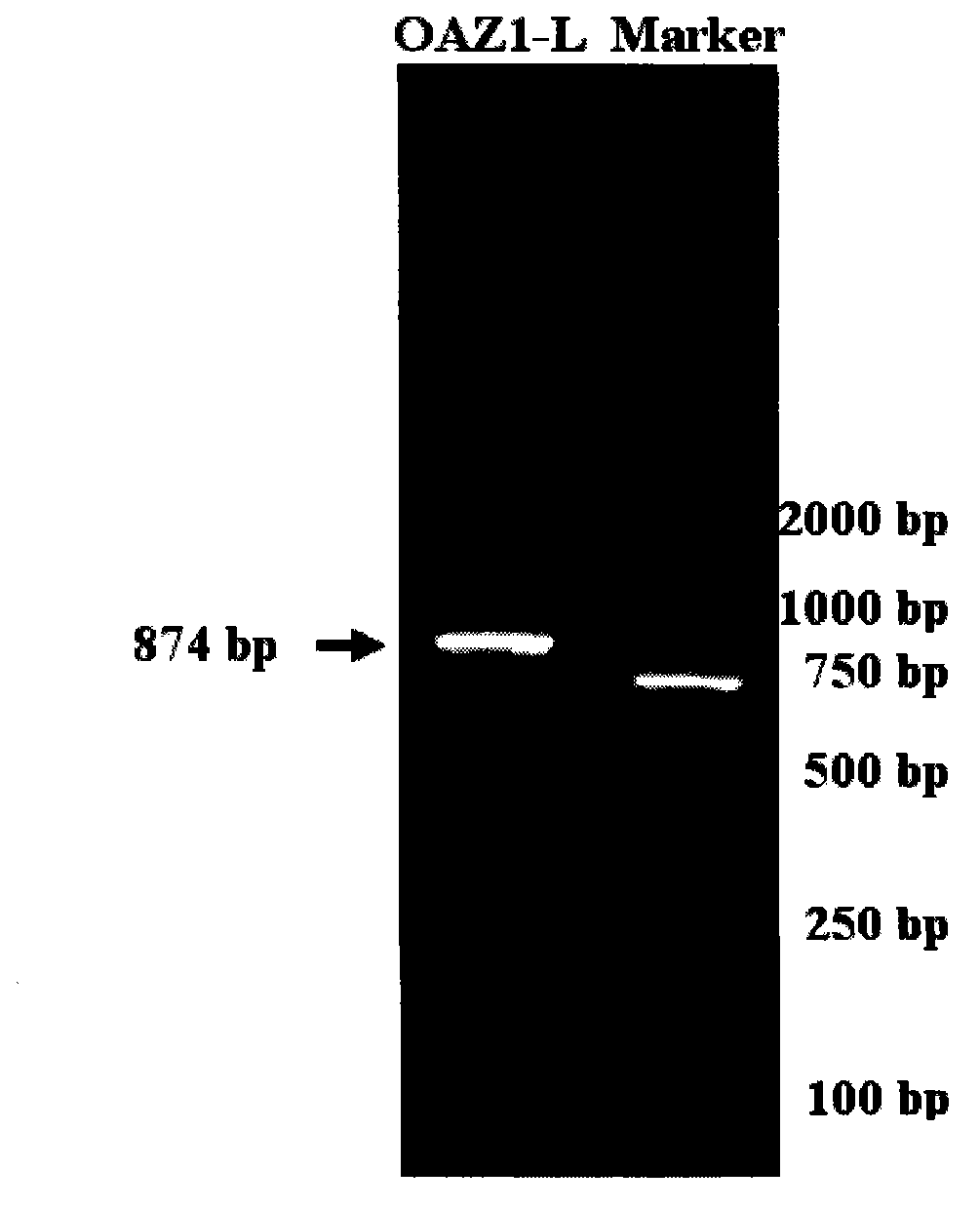
[0023] 2. Design primers for OAZ1-L gene, the length is 874bp;
[0024] Forward: TGCGGGGTGTTCAAGATGT
[0025] Reverse: GAGGGAGAGGACCTGCAAAC
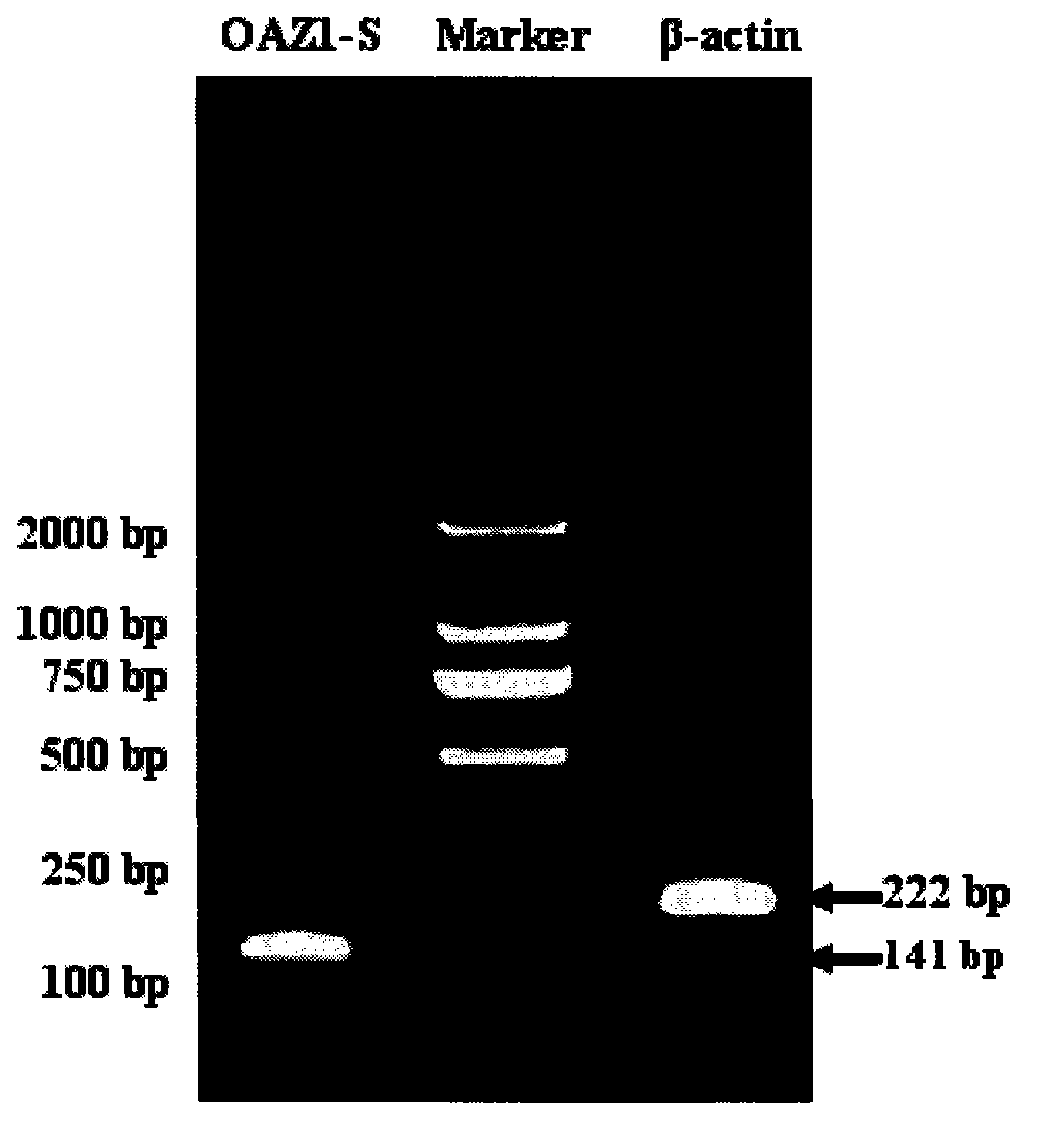
[0026] Design primers for OAZ1-S gene, the length is 141bp;
[0027] Forward: ACTTCAGGAACCCTCGCATCAACT
[0028] Reverse: GCTGCCCTCATCTTTCTAATACGG
[0029] Design primers for β-actin gene, the length is 222bp;
[0030] Forward: GCGGCATGCCACACCGTGCCCATCTATGAG
[0031] Reverse: GCGAAGCTTGGCCATCTCCTGCTCGAAGT
[0032] 3. Obtain the full-length OAZ1 sequence through RT-PCR amplification reaction, recovery and purification of PCR products, preparation of DH5α competent cells, ligation of target genes, transformation of ligated products, screening of positive colonies, and bacterial liquid seq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com