Derivatives of pesticide nereistoxin and preparation method of derivatives
A technology for nereid toxin and derivatives, which is applied in the preparation of organic compounds, amino hydroxy compounds, and thioether preparations, etc., can solve problems such as unscientific use, achieve low environmental residues, improve economic benefits, and avoid biological enrichment. and food chain accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
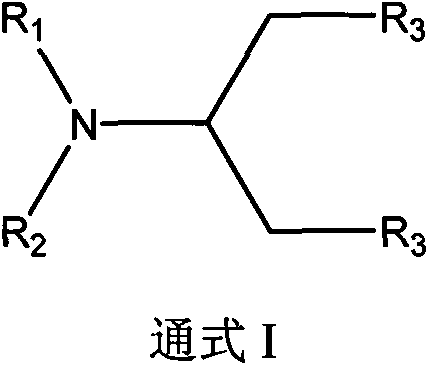
[0031] Embodiment 1 is a nereistin derivative whose structural formula is general formula I and its preparation method:
[0032] The specific method is as follows:
[0033] (1) Synthesis of 2-dimethylamino-1,3-propanediol
[0034] Add 52.3g of formic acid solution (aq, 88%, 1mol) into the round-bottomed flask, after ice bathing for a period of time, under stirring, slowly add 9.1g of serinol (0.1mol), and dropwise add 18.4g of it after the addition is complete Formaldehyde solution (aq, 37%, 0.22mol), was stirred at 60°C for 3 hours, at which time almost no gas was generated, and stirred at 90°C for 6 hours. Then the excess formic acid and most of the water were evaporated under reduced pressure at 70°C, the residue was dissolved in an appropriate amount of ethyl acetate, and Na 2 SO 4 Dry overnight, filter, discard the filter residue, and evaporate the filtrate to give 11.66 g of light yellow oily liquid, yield: 98%.
[0035] (2) Synthesis of 2-diethylamino-1,3-propanedio...
Embodiment 2
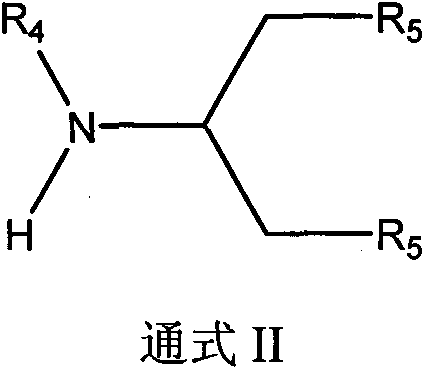
[0087] Embodiment 2 is a nereistin derivative whose structural formula is general formula II and its preparation method:
[0088] The specific method is as follows:
[0089] (1) Preparation of 2-chloro-5-methylthiazole
[0090] Put 8g of 2-amino-5-methylthiazole in a 100mL flask, add 32mL of concentrated hydrochloric acid, cool in an ice-water bath to 0°C, and slowly add NaNO 2 Solution (6g dissolved in 12mLH 2 O), stirred at 0°C for 3 hours, then at 80°C for 3 hours. Slowly cool to room temperature, add dichloromethane for extraction, discard the aqueous phase, and repeat the extraction three times. The organic phases were combined, dried overnight over anhydrous sodium sulfate, filtered, and the solvent was evaporated to give 6.63 g of an oil. The content is 90%.
[0091] (2) Preparation of 2-chloro-5-bromomethylthiazole
[0092] The 2-chloro-5-methylthiazole (6.63g) prepared above was dissolved in 60mLCCl 4 Add 9.76g of N-bromodibutyrimide and a catalytic amount of (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com