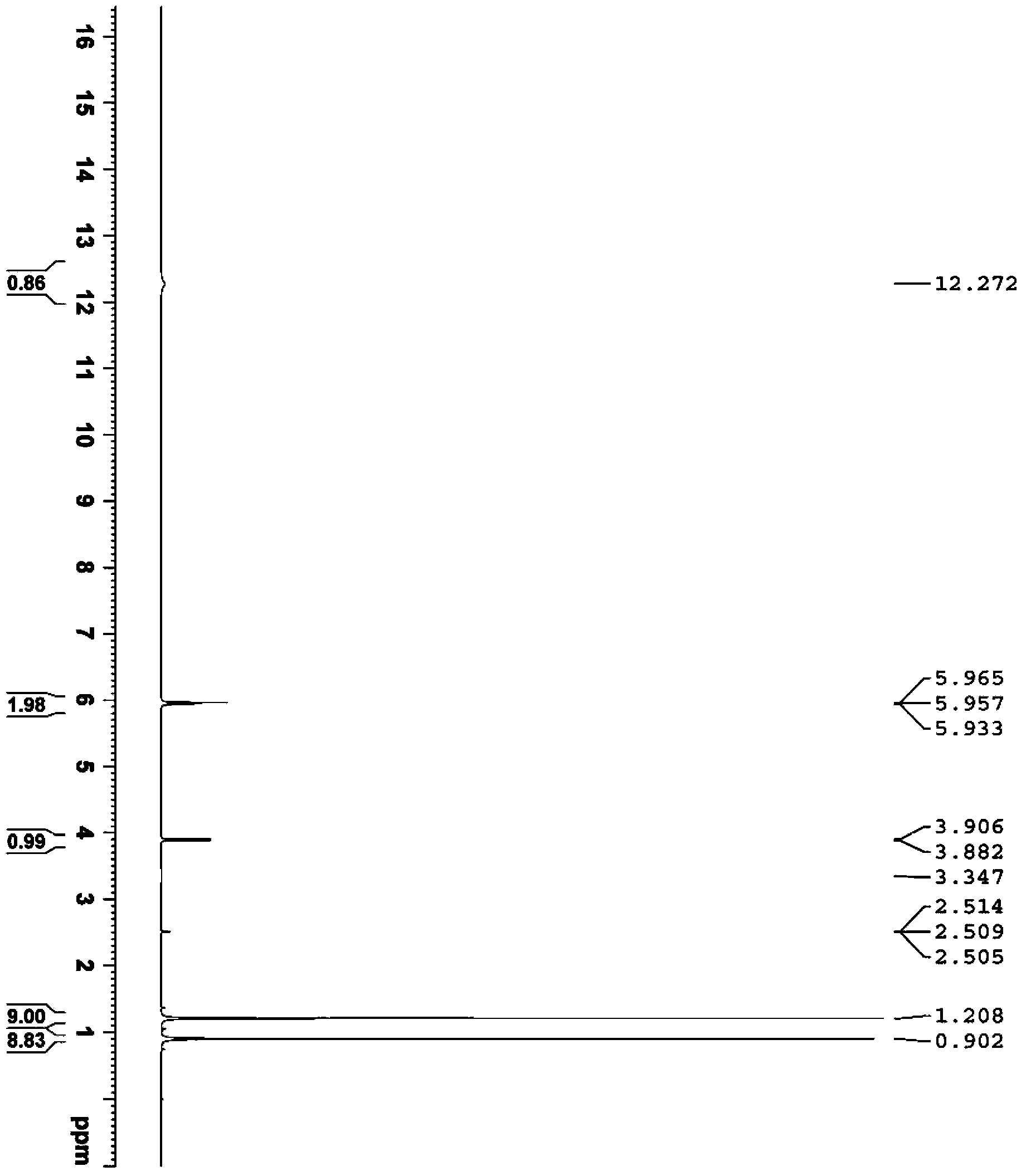One-pot method for synthesizing n-tert-butylaminocarbonyl-3-methyl-l-valine
A technology of tert-butylaminocarbonyl and synthetic method, which is applied in the field of drug synthesis, can solve the problems of affecting the yield of final product, greatly affecting the pH, and long synthesis time, and achieves shortened reaction cycle, controllable conditions, and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
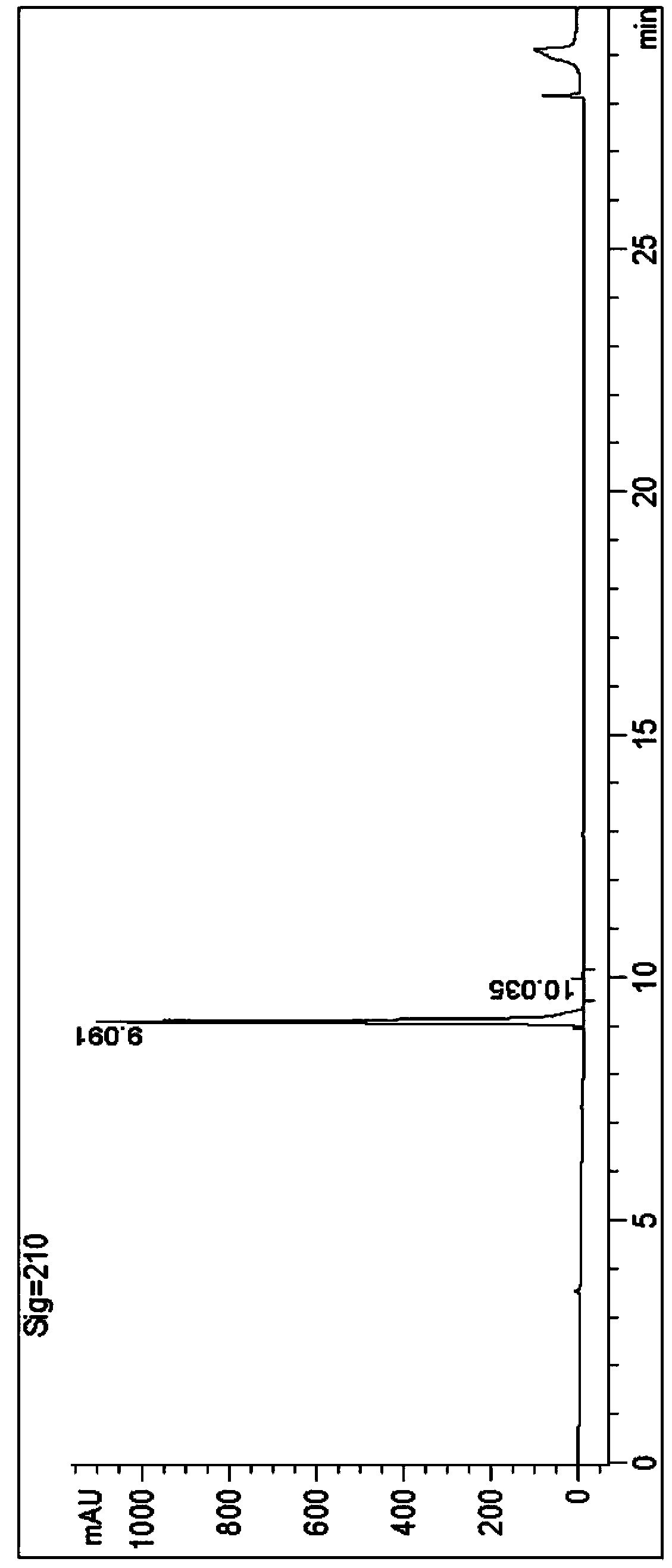
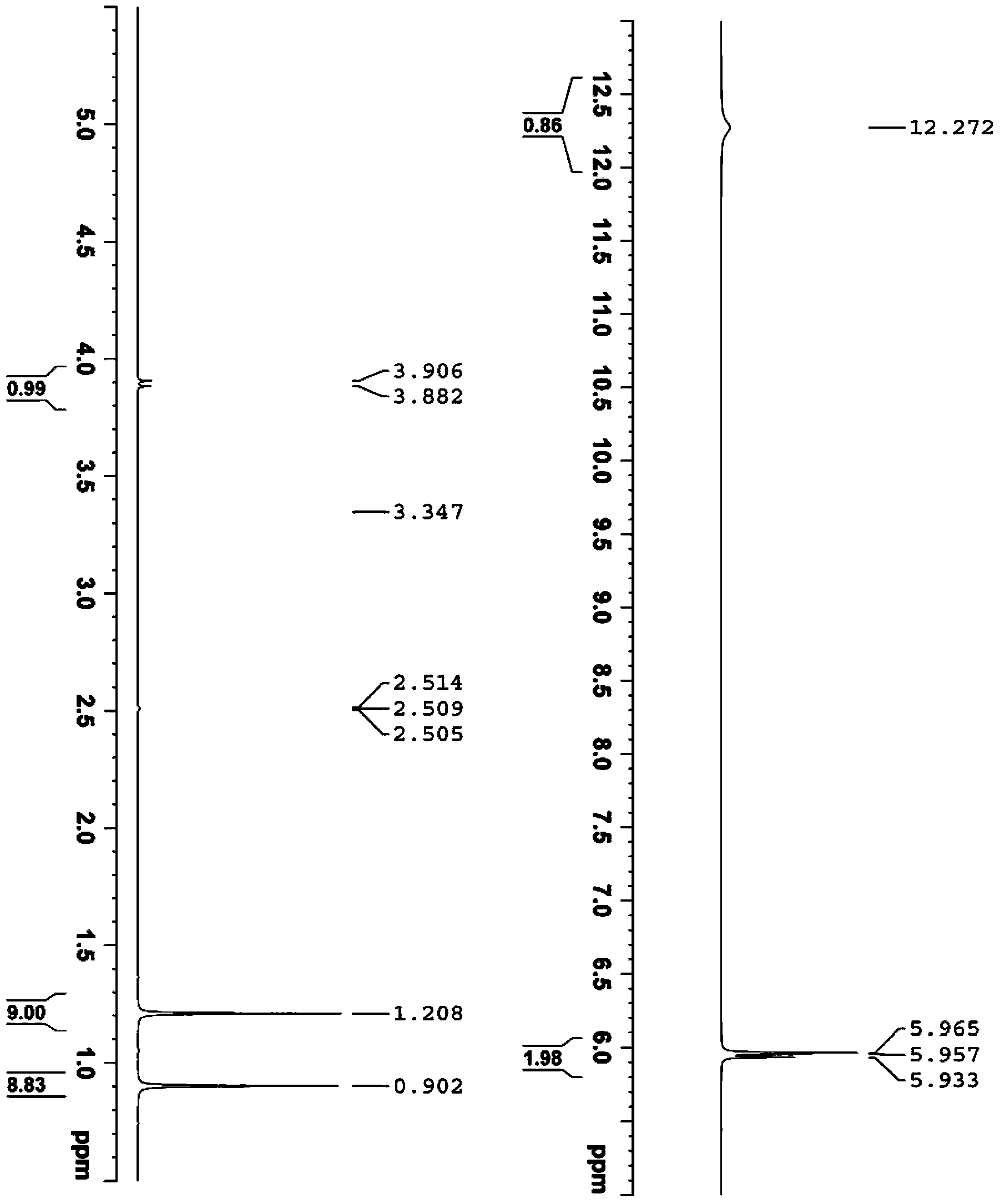
Image
Examples
Embodiment 1
[0036] In a 500mL reaction flask, dissolve 36.5g of tert-butylamine in 150mL of dichloromethane to form a reaction solution, cool the reaction solution to 0°C, first add 81g of N,N'-carbonyldiimidazole, and react for 30 minutes under stirring until the reaction is complete as detected by TLC; Then add 65.5g of L-tert-leucine to the reaction solution, and react for 30 minutes under stirring until the reaction is complete as detected by TLC; then add the reaction solution to ice water, extract with 150mL of dichloromethane, and concentrate under reduced pressure at 35°C Obtain a white solid; get 200g of the gained white solid and use n-heptane to make a slurry, vacuum-dry to obtain 113g of the final product N-tert-butylaminocarbonyl-3-methyl-L-valine after suction filtration, the purity is 99.6%, and the yield is 98%.
Embodiment 2
[0038] In a 100mL reaction flask, 73g of tert-butylamine was dissolved in 300mL of tetrahydrofuran to form a reaction solution. When the reaction solution was cooled to 5°C, 162g of N,N'-carbonyldiimidazole was added and stirred for 40 minutes until the reaction was detected by TLC; Add amino acid to the reaction solution, stir for 40 minutes until the reaction is complete as detected by TLC, then add the reaction solution to ice water, extract with 300mL tetrahydrofuran, concentrate under reduced pressure at 40°C to obtain a white solid; beat the obtained white solid with methanol , vacuum-dried after suction filtration to obtain 225 g of the final product N-tert-butylaminocarbonyl-3-methyl-L-valine, with a purity of 99.5% and a yield of 97.6%.
Embodiment 3
[0040] In a 250mL reaction flask, 18g of tert-butylamine was dissolved in 70mL of methanol to form a reaction solution. When the reaction solution was cooled to -5°C, 40g of N,N'-carbonyldiimidazole was added and stirred for 20min until the reaction was detected by TLC; then 30gL-tert- Leucine was added to the reaction solution, stirred for 20 minutes until the reaction was detected by TLC, then the reaction solution was added to ice water, extracted with 70 mL of n-butanol, and concentrated under reduced pressure at 30°C to obtain a white solid; the obtained white solid Slurry with ether, vacuum-dry after suction filtration to obtain 55.2 g of the final product N-tert-butylaminocarbonyl-3-methyl-L-valine, with a purity of 99.2% and a yield of 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com