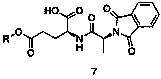
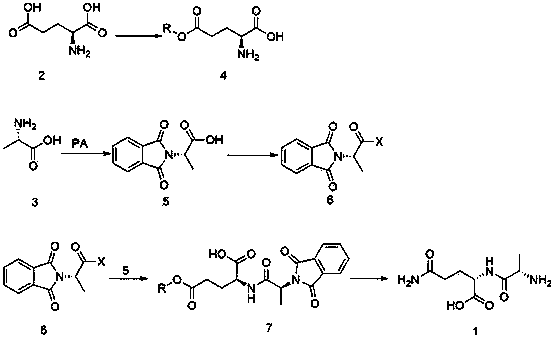
New preparation method of L-alanyl-L-glutamine
A technology of glutamine and alanyl, applied in the direction of peptides, etc., can solve the problems of high price and high price of L-alanyl-L-glutamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment L-1
[0018] ? The preparation of embodiment L-1 methyl glutamate
[0019] ??Add anhydrous methanol (1280.0g, 40.0mol, 33.3eq) into a 3000mL three-necked flask, add (180.0g, 1.2mol, 1.0eq) L-glutamic acid to the reaction kettle under stirring, and cool to 0 in an ice-water bath -5°C, add concentrated sulfuric acid (166.0g, 1.7mol, 1.4eq) dropwise and then rise to the reaction temperature of 20°C, maintain the temperature for 3 hours, the liquid phase detection of L-glutamic acid reaction is complete; cool to 0~ 5°C, add triethylamine dropwise to pH = 7.0-8.0, keep warm at 0°C for crystallization for 2 hours; filter, wash the filter cake with an appropriate amount of anhydrous methanol, and dry at 50°C for 2 hours to obtain 180.0 g of L-methyl glutamate , yield: 92.0%, purity 98.8% (HPLC peak area normalization method).
Embodiment L-2
[0020] The preparation of embodiment L-2 ethyl glutamate
[0021] ??Add absolute ethanol (2208.0g, 48.0mol, 40eq) into a 5000mL three-neck flask, add (180.0g, 1.2mol, 1.0eq) L-glutamic acid into the reaction kettle under stirring, and cool in an ice-water bath to 0~ 5°C, add concentrated sulfuric acid (166.0g, 1.7mol, 1.4eq) dropwise and then rise to the reaction temperature of 20°C, maintain the temperature for 3 hours, the liquid phase detection of L-glutamic acid reaction is complete; cool down to 0-5 ℃, add triethylamine dropwise to pH = 7.0-8.0, keep crystallization at 0 ℃ for 2 hours; filter, wash the filter cake with an appropriate amount of anhydrous methanol, and dry at 50 ℃ for 2 hours to obtain 193.0 g of ethyl L-glutamate. Yield: 93.0%, purity 97.8% (HPLC area normalization method).
Embodiment 3
[0022] ??Example 3 Preparation of phthaloyl-L-alanine
[0023] ??Put phthalic anhydride (148.0g, 1.0mol, 1.0eq) and L-alanine (89.0g, 1.0mol, 1.0eq) into a 500mL three-necked flask, heat up, and as the temperature of the reaction material rises , the material slowly melted into a liquid state, and started stirring at the same time. When the temperature in the kettle reached 175°C, keep the temperature for 25 minutes. %, purity: 99.3% (HPLC peak area normalization method).
[0024] ?? Embodiment? 4 Preparation of phthaloyl-L-alanyl chloride
[0025] ??Put toluene (1200.0g, 13.0mol, 18.8eq) and phthalyl L-alanine (151 g, 0.69 mol, 1eq) into a 2000 mL three-neck flask, stir in a water bath to raise the temperature, and control the temperature at 60°C Next, add thionyl chloride (88.0 g, 0.74 mol, 1.1 eq) dropwise, keep warm at 60-65°C for 3 hours, concentrate toluene under reduced pressure, the weight of the oil is 162.0 g, yield: 99.0%, purity: 99.0% (HPLC peak area normalizat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com