Synthetic method of 7-amino-3-chloro-3-cephem-4-carboxylic acid
A synthetic method, cephalosporin technology, applied in the field of drug synthesis, can solve the problems of high cost, by-products, and many steps, and achieve the effect of low cost, good quality, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
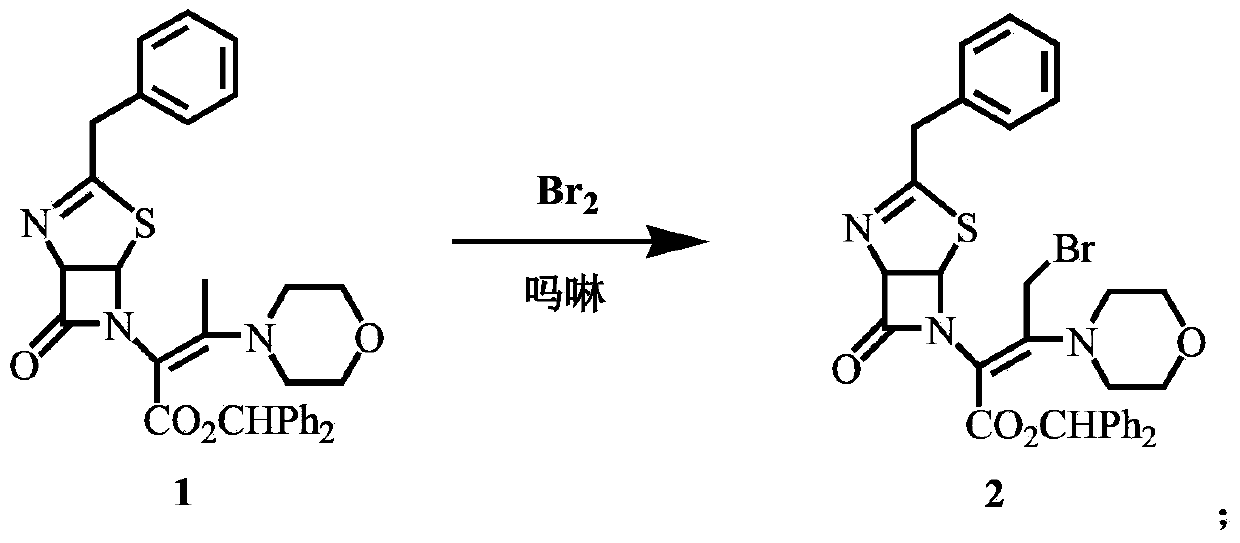
[0038] Synthesis of compound 2
[0039] Add 150mL of dichloromethane and 22g of compound 1 (40mmol) to a 250mL four-necked bottle, cool down to -10°C, add 3.83g (44mmol) of morpholine, add 6.4g (40mmol) of liquid bromine dropwise within 30min, and the addition is complete ,-20°C and continued to stir for 30 minutes to obtain a solution of compound 2.
Embodiment 2
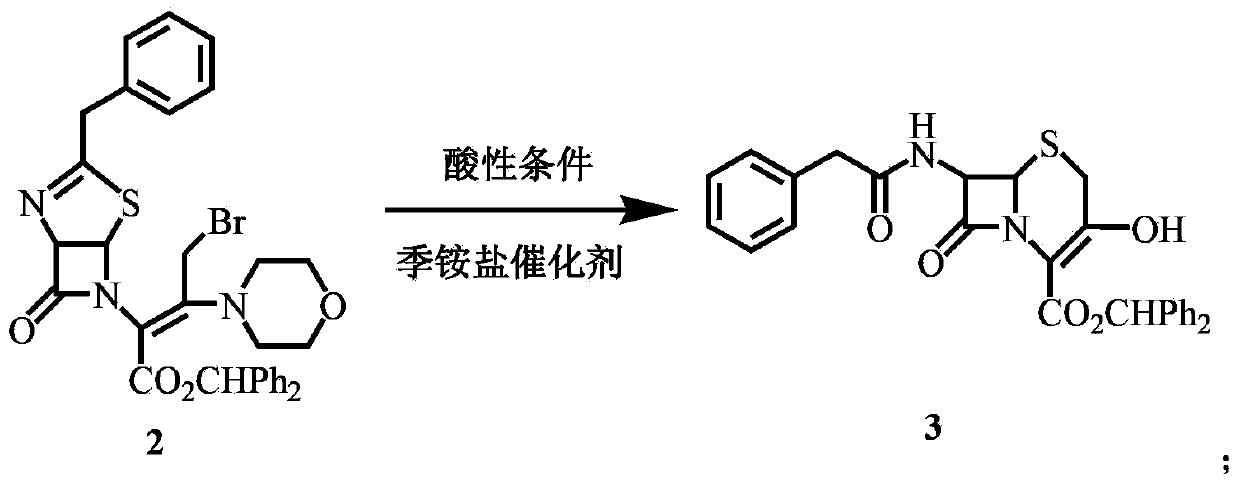
[0041] Synthesis of 3‐OH cephalosporin (compound 3)
[0042] Add 32 mL of 8.5% sulfuric acid aqueous solution to the solution of compound 2 to make the pH around 0.7, add 160 mL of methanol and 1.0 g of trioctylmethylammonium chloride, and react at 15°C for 12 hours (monitoring the reaction by TLC). Add 20 mL of water, stir at room temperature for 5 min, separate the liquids, wash the organic phase twice with 50 mL of 2% dilute sulfuric acid solution, extract the water phase with 150 mL of dichloromethane, combine the organic phases, wash with saturated brine, and concentrate the organic phase under reduced pressure at 30°C. phase, after the content becomes viscous, add 80 mL of methanol, heat to dissolve, cool down and crystallize, stir at 0-5°C for 1 hour, filter with suction, and wash the filter cake with 40 mL of ice methanol to obtain 19.5 g of a light yellow solid with a melting point of 141.4-142.7 °C, the yield was 97.5%.
Embodiment 3
[0047] Synthesis of 7‐phenylacetamido‐3‐chloro‐3‐cephronoic acid benzhydryl ester (compound 4)
[0048] Add 120mL of N,N‐dimethylformamide to a 250mL four-neck bottle, add 8.0g of PCl dropwise at 0~5℃3 (58.3mmol), the dropwise addition was completed, stirred and reacted at 20°C for 1h, added 20g (40.0mmol) of 3‐OH cephalosporin prepared in the previous step, and reacted at 20°C for 3h (monitored by TLC). After the reaction is complete, pour the reaction solution into 300 mL of water pre-cooled to below 5°C, stir at 0-5°C for 1 hour, filter with suction, wash the filter cake with aqueous sodium bicarbonate solution and distilled water, and dry under vacuum at 50°C to obtain a yellow solid 7‐ 20.5 g of benzhydryl phenylacetamido-3-chloro-3-cephemate, the melting point is 171.2-172.3°C, and the yield is 98.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com