Method for synthesizing trioxymethylene
A synthesis method and trioxymethylene technology, which are applied in chemical recycling, organic chemistry and other directions, can solve the problems of high cost of ionic liquid and low trioxymethylene yield, and achieve a good promotion effect, remarkable promotion effect and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of embodiment 1, paraformaldehyde
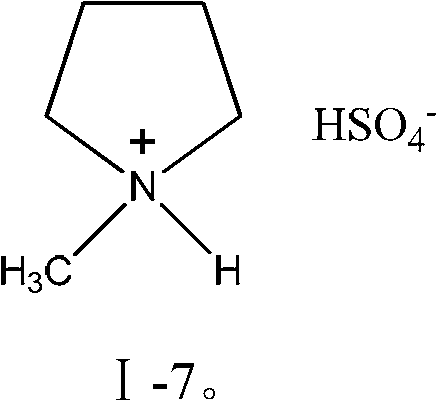
[0028] A rectification reaction device is adopted, wherein the rectification section is a glass packed tower with an inner diameter of 30mm and a height of 300mm, and the reaction kettle is a 250mL glass four-necked flask. Add 137.6g 60.0wt.% formaldehyde solution in the reactor, heat to boiling, then add successively the ionic liquid shown in 2.75g catalyst formula I-1 (being 2.0% of formaldehyde solution quality) and 1.38g accelerator LaCl 3 (1.0% of the quality of the formaldehyde aqueous solution); Heating total reflux reaction, the temperature at the bottom of the reactor is controlled at 95 ° C, the temperature at the top of the tower is 90 ° C, and the reaction is 90 minutes. The reaction solution in the reactor is taken, and the content of each component is analyzed by chromatography.
[0029] The content of paraformaldehyde in the reaction solution was 2.51wt.%, the content of methanol was 0.39wt.%, and impuriti...
Embodiment 2
[0030] The synthesis of embodiment 2, paraformaldehyde
[0031] With embodiment 1, get the formaldehyde aqueous solution of 137.2g 59.7wt.%, add ionic liquid shown in 2.6g catalyst formula I-2 (being 1.90% of formaldehyde aqueous solution quality), 1.37g promotor LaCl 3 (1.0% of the mass of the formaldehyde aqueous solution); wherein the temperature at the bottom of the reaction tank was controlled at 102° C., the temperature at the top of the tower was 95° C., and the reaction was performed for 3 hours.
[0032] According to chromatographic analysis, the content of paraformaldehyde in the reaction kettle liquid was 2.72wt.%, and the content of methanol was 0.62wt.%, and impurities such as methylal and methyl formate were not detected. In contrast, under the same reaction conditions, 60.0wt.% concentrated formaldehyde was used to add 2.0wt.% of the ionic liquid shown in the catalyst formula I-2, but no accelerator LaCl was added 3 , the content of paraformaldehyde in the reac...
Embodiment 3
[0033] The synthesis of embodiment 3, paraformaldehyde
[0034] With embodiment 1, get the formaldehyde aqueous solution of 133.7g 60.0wt.%, add the ionic liquid shown in 2.6g catalyst formula I-3 (being 1.94% of formaldehyde aqueous solution quality), 1.32g accelerator CuCl 2 (1.0% of the mass of the formaldehyde aqueous solution), wherein the temperature at the bottom of the reaction tank was controlled at 98° C., the temperature at the top of the tower was 92° C., and the reaction was performed for 3 hours.
[0035] According to chromatographic analysis, the content of paraformaldehyde in the reaction kettle liquid was 2.69wt.%, and the content of methanol was 0.52wt.%, and impurities such as methylal and methyl formate were not detected. In contrast, under the same reaction conditions, 60.0wt.% concentrated formaldehyde was used to add 2.0wt.% catalyst ionic liquid shown in formula I-3, but no accelerator CuCl was added 2 , the content of paraformaldehyde in the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com