Preparation method of sulfonyl azide compounds
A technology for sulfonyl azide compounds, applied in the field of preparation of sulfonyl azide compounds, to achieve the effects of safe and controllable operation, mild process conditions, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
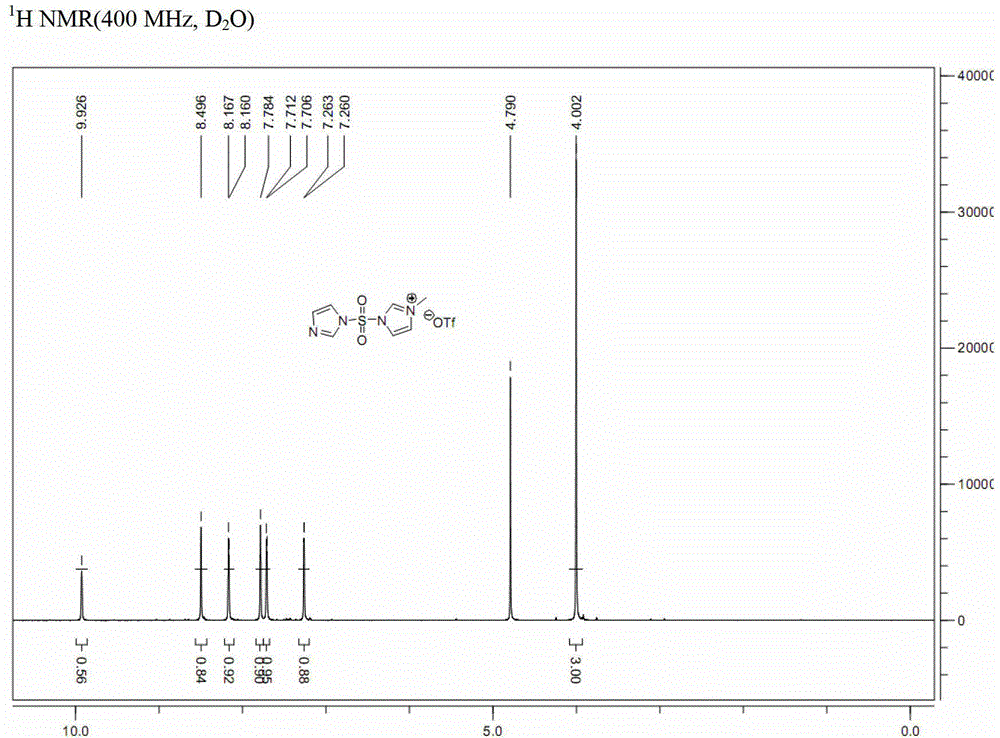
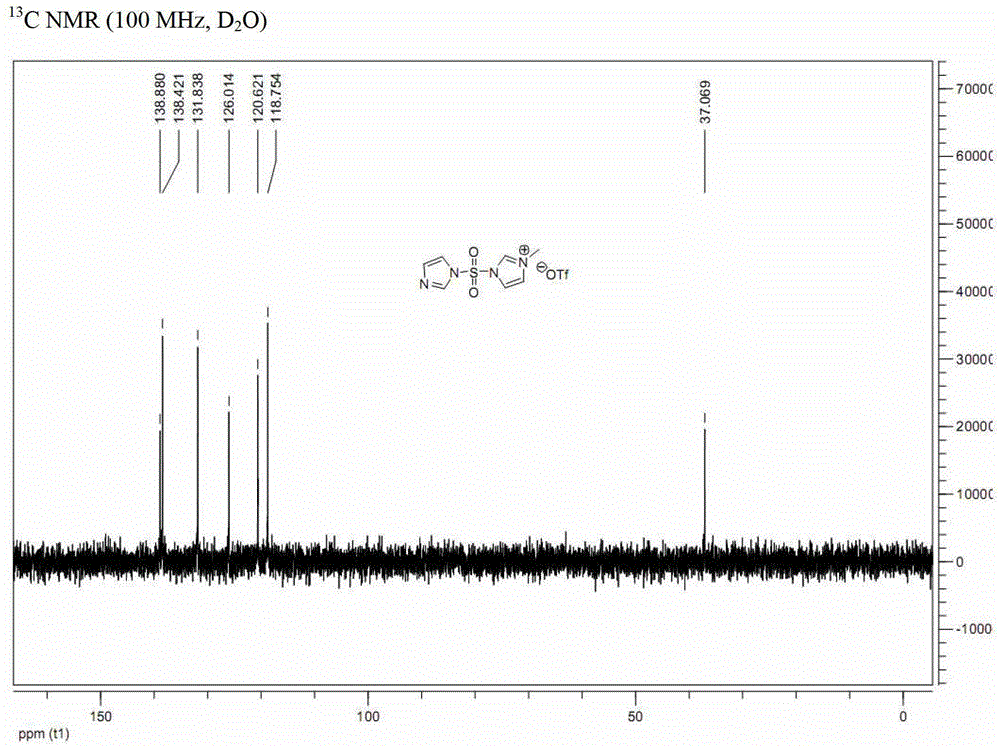
[0041] Embodiment 1: a kind of preparation method of formula (I-A) imidazole-1-sulfonyl azide, comprises the steps:
[0042]
[0043] a. Dissolve N,N'-sulfonyldiimidazole (compound of formula (II-A); 100g, 0.50mol) in dichloromethane (1L), and add methyl trifluoromethanesulfonate dropwise at 0°C (50.9mL, 0.45mol), and then continue to react at 0°C for 2 hours, and the resulting solid is filtered, washed with dichloromethane, and vacuum-dried to obtain 163g of white solid powder (analyzed by nuclear magnetic resonance, which is 3-(imidazole-1 -sulfonyl)-1-methyl-3H-imidazole-1-trifluoromethanesulfonate, compound III-A), (yield 100%);
[0044]
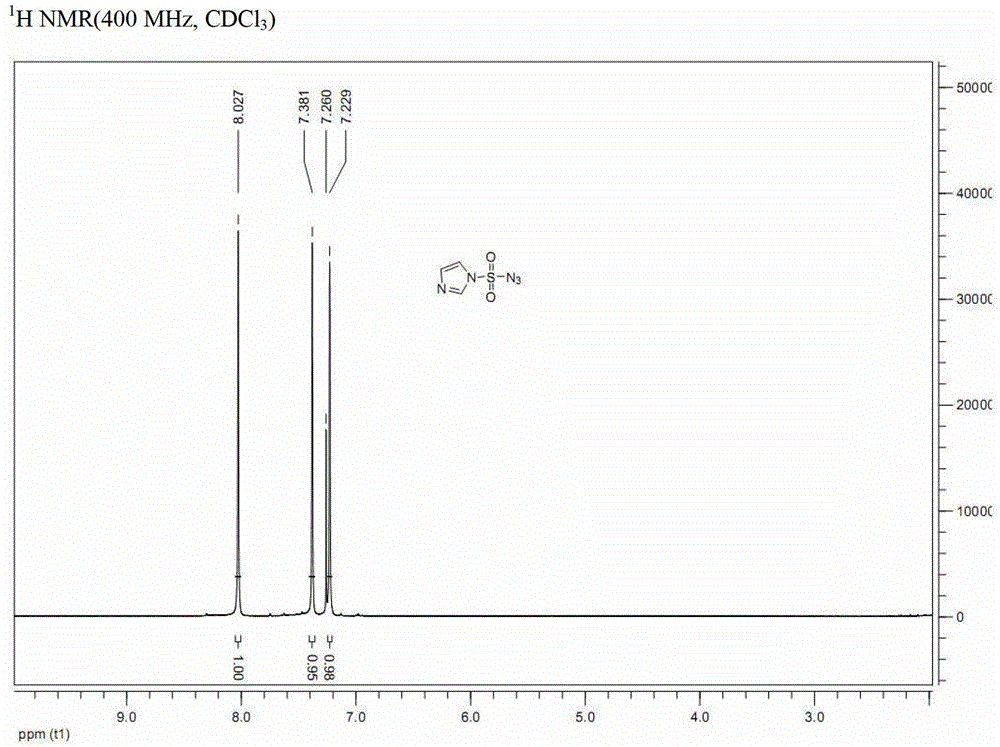
[0045] b. Dissolve the compound of formula (III-A) (163g, 0.45mol) in ethyl acetate / water (1:1, 1080mL), add sodium azide (58.5g, 0.9mol) at 0°C, and the reaction solution Stir at 0° C. for 1.5 hours. After the reaction is complete, imidazole-1-sulfonyl azide (compound I-A after nuclear magnetic resonance analysis) is obtained.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com