Caffeoyl substituted pentacyclic triterpenoid derivatives and purpose thereof
A pentacyclic triterpenoid and caffeoyl technology, applied in the field of medicine, can solve the problems of weak clinical efficacy results of neuroprotective agents, no expected effect, and clinical application of large toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
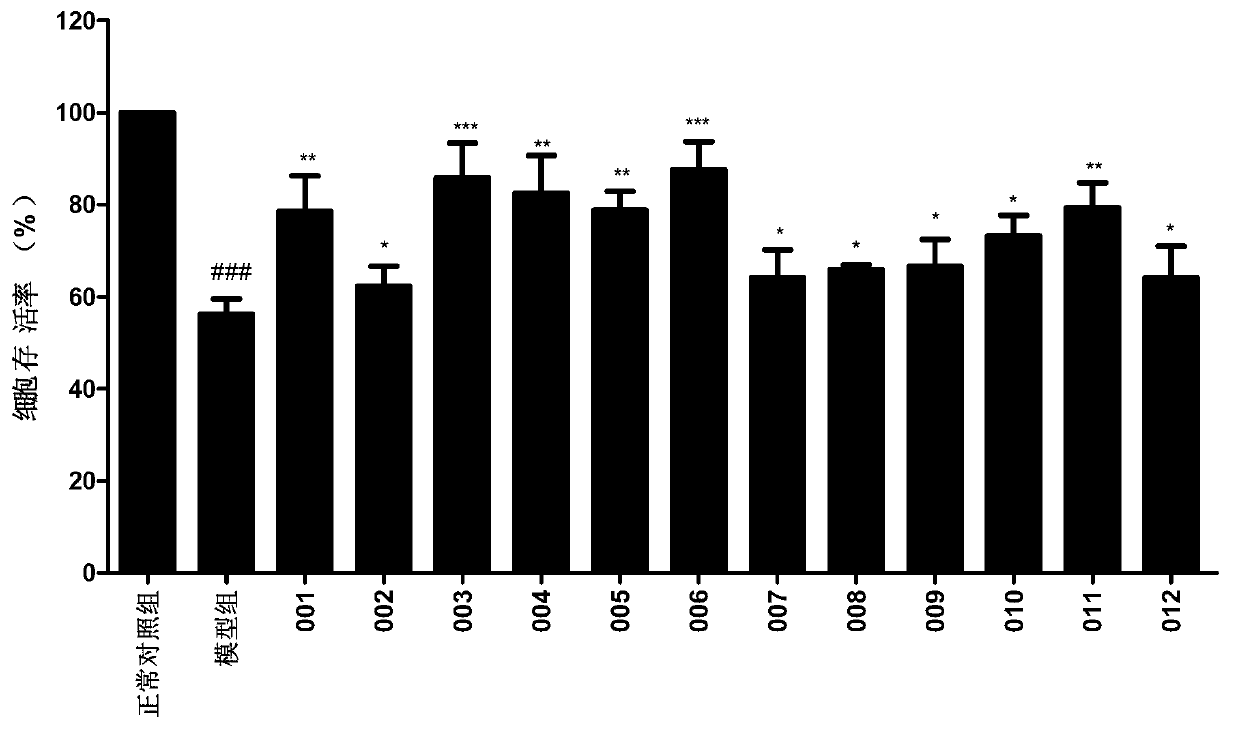
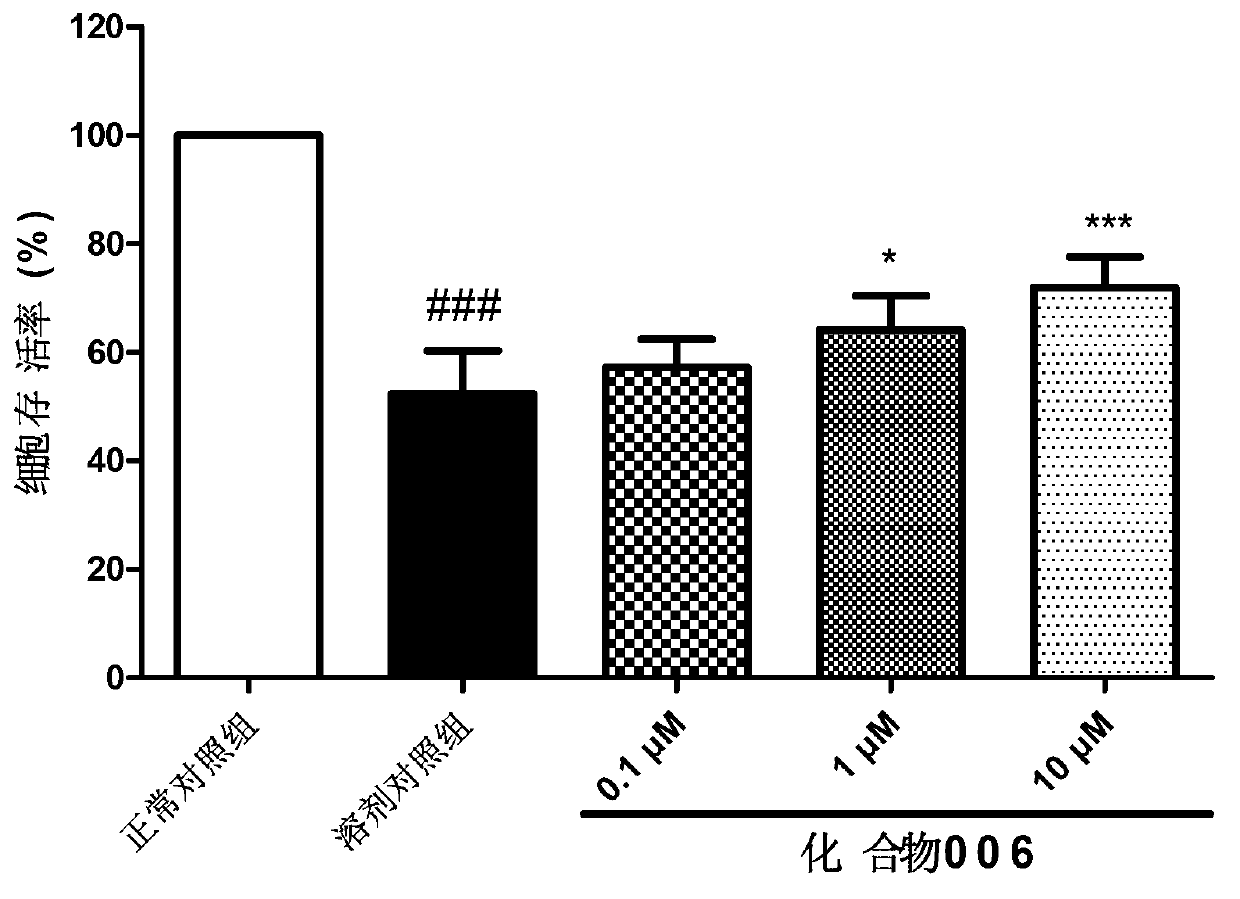
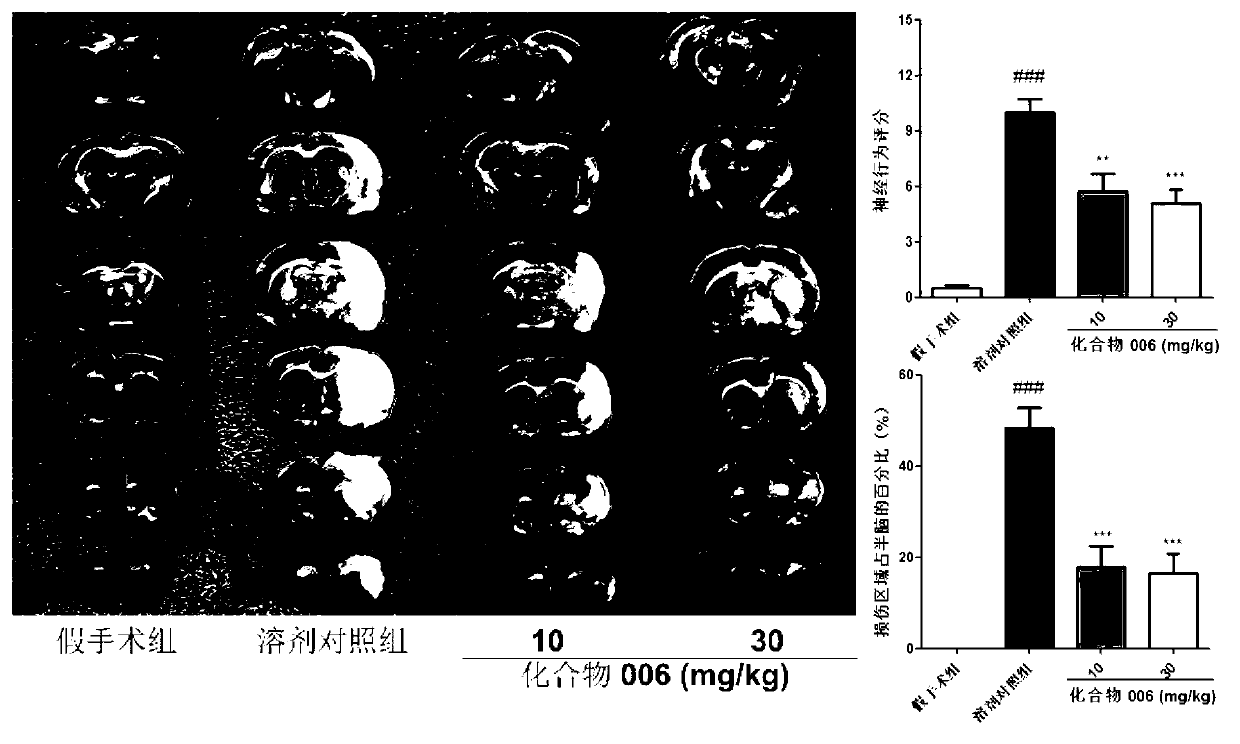
Image
Examples
Embodiment 1
[0172] Example 1: Preparation of Compounds 001-005
[0173] The root bark (20Kg) of C.orbiculatus (C.orbiculatus, collected from Huaihua, Hunan Province, in April 2009) dried in the shade and crushed was extracted by percolation with 95% ethanol at room temperature (30L×3), and the extract was concentrated under reduced pressure to obtain ethanol extract . The ethanol extract was suspended and dissolved in 10% methanol (1.5L), and extracted three times with ethyl acetate (1.5L×3) to obtain the extract of ethyl acetate (400g). Ethyl acetate extract was subjected to normal phase separation and gradient elution with petroleum ether-ethyl acetate (10:1,6:1,4:1,2:1,1:1) to obtain 4 components (Fr.1 -4). Among them, Fr.2 was subjected to reverse phase, normal phase, LH-20, HW-40 repeated column chromatography to obtain compound 002 and compound 004; Fr.3 was subjected to reversed phase, normal phase, LH-20, HW-40 repeated column chromatography Compounds 001, 003 and 005 were obta...
Embodiment 2
[0179] Example 2: Synthesis of 3β-hydroxyl-28-caffeoyloxy-20(29)-ene-lupine (006)
[0180]
[0181] Under ice bath conditions, to betulin (200mg, 452.5mmol) in DCM (CH 2 Cl 2 ) solution (20 mL), add freshly prepared caffeoyl chloride (179.2 mg, 905.0 mmol), and stir at room temperature. After the completion of the reaction as detected by TLC, add saturated NaHCO 3 The solution (50 mL) was quenched. Extracted with DCM (50mL x3), washed with saturated brine, anhydrous Na 2 SO 4 dry. The residue after evaporating DCM to dryness was purified by a normal-phase silica gel column, eluting with petroleum ether:ethyl acetate (5:1), to obtain a white powdery solid, compound 006, 164 mg, yield 60%. 1 H NMR (500MHz, deuterated chloroform): δ0.77(s,3H),0.82(s,3H),0.97(s,3H),0.98(s,3H),1.04(s,3H),1.69(s ,3H),0.68-2.00(m,25H),2.46(dt,J=10.8,5.7Hz,1H),2.68(m,1H),3.21(dd,J=11.3,4.6Hz,1H),3.97( d,J=11.0Hz,1H),4.37(d,J=11.0Hz,1H),4.60(s,1H),4.70(s,1H),6.28(d,J=16.0Hz,1H),6.45( br s,1...
Embodiment 3
[0182] Example 3: Synthesis of 3β-caffeoyloxy-20(29)-en-28-ol-lupinane (007).
[0183]
[0184] Step 1: To a freshly prepared solution of 3,4-diacetoxycaffeoyl chloride in dry DCM (5ml) with stirring in an ice bath, was added 3β-hydroxy-20(29)-ene-28-acetoxy- Lupinane (218mg, 0.45mmol), pyridine (113mg, 1.35g), stirred at room temperature for 4h. After the reaction solution was concentrated, it was directly separated by normal phase column chromatography, eluting with petroleum ether: ethyl acetate (10:1), to obtain compound 3β-(3,4-diacetoxy-caffeoyloxy)-20(29 )-ene-28-acetoxy-lupin (230 mg, 0.32, 71% yield).
[0185] Step 2: To the methanol (3mL ) solution was added a catalytic amount of sodium metal, stirred at room temperature for 30min, TLC detected no raw material point, added hydrochloric acid solution (1M, 10mL), stirred at room temperature for 30min, extracted with ethyl acetate (10mLx3), and the extract was decompressed to remove the solvent Afterwards, it was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com