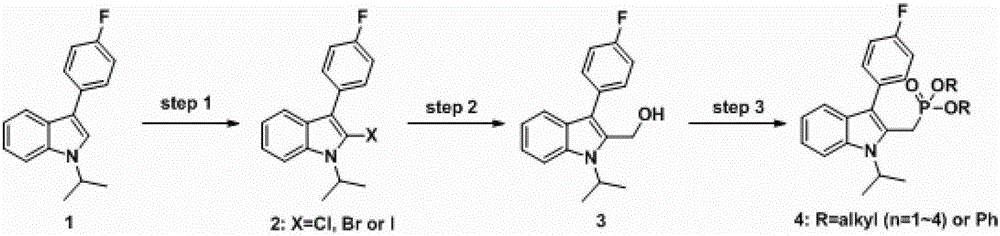
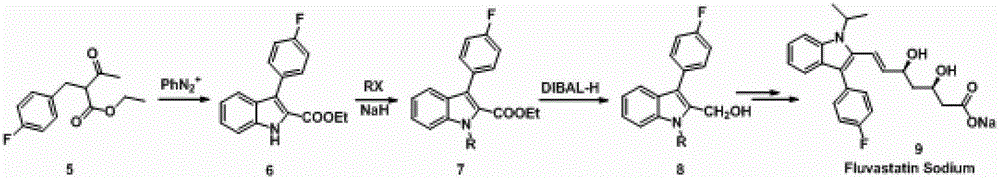
A kind of method for preparing fluvastatin key intermediate
A fluvastatin and intermediate technology, applied in the field of organic synthetic pharmaceuticals, can solve the problems of high cost, low total yield, long reaction steps, etc., and achieve the effects of reducing raw material costs, short synthetic routes, and good chemical selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: a kind of method for preparing fluvastatin key intermediate, is characterized in that concrete preparation steps are as follows:
[0033] (1) Halogenation reaction: Control the temperature at 25±5°C, add 504.0kg (5mL / g) of 1,2-dichloroethane into a 2000L reactor, and the main raw material 3-(4-fluorophenyl)-1 - Isopropyl-1H-indole 80 kg (315.8 mol, 1.0 equiv.) and N-bromosuccinimide 67.5 kg (379.0 mol, 1.2 equiv.). The system was heated to reflux for reaction, and detected by HPLC until the reaction was completed. The system was pressed into 480.0 kg of saturated aqueous sodium bisulfite solution (5 mL / g) to terminate the reaction. Stand still, separate the liquid, concentrate the organic phase, and then add 126.4kg (2mL / g) of ethanol to recrystallize to obtain the halogenated product 2-bromo-3-(4-fluorophenyl)-1-isopropyl-1H-indole 89.2 kg, the liquid phase purity was 96.0%, and the yield was 85.0%.
[0034] (2) Hydroxymethylation reaction: Control the...
Embodiment 2
[0036] Embodiment 2: a kind of method for preparing fluvastatin key intermediate, is characterized in that concrete preparation steps are as follows:
[0037] (1) Halogenation reaction: Control the temperature at 25±5°C, add 665.0kg (10mL / g) of dichloromethane to a 2000L reactor, and the main raw material 3-(4-fluorophenyl)-1-isopropyl -1H-indole 50kg (197.4mol, 1.0equiv.) and tribromopyridine 62.3kg (197.4mol, 1.0equiv.). The system was heated to reflux for reaction, followed by HPLC detection until the end of the reaction. Press the system into 600.0 kg (10 mL / g) of saturated aqueous sodium bisulfite solution to terminate the reaction. Stand still, separate the liquid, concentrate the organic phase, add methanol 197.5kg (5mL / g) to recrystallize to obtain the halogenated product 2-bromo-3-(4-fluorophenyl)-1-isopropyl-1H-indole 62.3 kg, the liquid phase purity was 99.0%, and the yield was 95.0%.
[0038] (2) Hydroxymethylation reaction: control the temperature at 25±5°C, ad...
Embodiment 3
[0040] Embodiment 3: a kind of method for preparing fluvastatin key intermediate, is characterized in that concrete preparation steps are as follows:
[0041] (1) Halogenation reaction: Control the temperature at 25±5°C, add 499.5kg (15mL / g) of chlorobenzene into a 2000L reactor, and the main raw material 3-(4-fluorophenyl)-1-isopropyl-1H - Indole 30 kg (118.4 mol, 1.0 equiv.) and bromine 28.4 kg (177.6 mol, 1.5 equiv.). The system was heated to reflux for reaction, followed by HPLC detection until the end of the reaction. Press the system into 540.0 kg (15 mL / g) of saturated aqueous sodium bisulfite solution to terminate. Stand still, separate the liquid, concentrate the organic phase, and then add 355.5kg (15mL / g) of isopropanol for recrystallization to obtain the halide 2-bromo-3-(4-fluorophenyl)-1-isopropyl-1H-ind Indole (Compound 2) 35.4kg, HPLC purity 97.0%, yield 90.0%.
[0042] (2) Hydroxymethylation reaction: Control the temperature at 25±5°C, add tetrahydrofuran 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com