Direct bilirubin detection reagent
A technology for detecting reagents and bilirubin, applied in the field of medical testing, can solve the problems of insufficient automatic biochemical testing equipment and facilities, lack of testing professionals, etc., and achieve the effects of good sensitivity, low price and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Diluent:
[0041] Tris hydroxymethyl amino buffer (pH7.0) 0.1mol / L
[0042] TWEEN80 5%
[0043] Proclin300 0.1%
[0044] Vitamin C oxidase 1KU / L
[0045] Reagent:
[0046] Phosphate buffer (pH4.0) 0.1mol / L
[0047] Sodium chloride 45g / L
[0048] Disodium EDTA 2g / L
[0049] Hydroxylamine Sulfate 2g / L
[0050] etidronate 2g / L
[0051] Sodium persulfate 100g / L
[0052] Sulfuric acid 0.1ml / L
[0053] Potassium sorbate 0.1g / L
[0054] Bovine serum albumin 5g / L.
Embodiment 2
[0056] Diluent:
[0057] Tris hydroxymethyl amino buffer (pH7.5) 0.5mol / L
[0058] TRITONX-100 5%
[0059] Methylparaben 1%
[0060] Vitamin C oxidase 2KU / L
[0061] Reagent:
[0062] 2-(N-morpholine)sodium ethanesulfonate buffer solution (pH3.0) 0.5mol / L
[0063] Sodium chloride 45g / L
[0064] Disodium EDTA 10g / L
[0065] Hydroxylamine Sulfate 5g / L
[0066] etidronate 5g / L
[0067] Sodium persulfate 150g / L
[0068] Sulfuric acid 1ml / L
[0069] Proclin300 1g / L
[0070] Trehalose 10g / L.
Embodiment 3
[0072] Diluent:
[0073] 3-Morpholinepropanesulfonic acid buffer (pH6.5) 1.0mol / L
[0074] SPAN20 10%
[0075] Sodium Benzoate 1%
[0076] Vitamin C oxidase 10KU / L
[0077] Reagent:
[0078] Sodium citrate buffer (pH5.0) 1mol / L
[0079] Sodium chloride 45g / L
[0080] Disodium EDTA 5g / L
[0081] Hydroxylamine Sulfate 10g / L
[0082] etidronate 10g / L
[0083] Sodium persulfate 50g / L
[0084] Sulfuric acid 1ml / L
[0085] Proclin300 1g / L
[0086] Fatty alcohol polyoxyethylene ether 10g / L.
[0087] The performance of the reagent obtained in Example 1 of the present invention is described below in conjunction with the table.
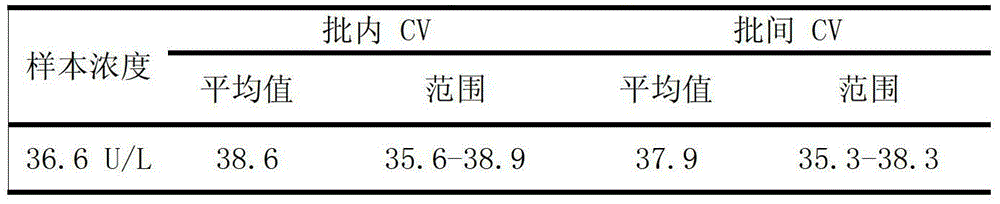
[0088] 1. Precision
[0089] Table 1. Precision evaluation results
[0090]
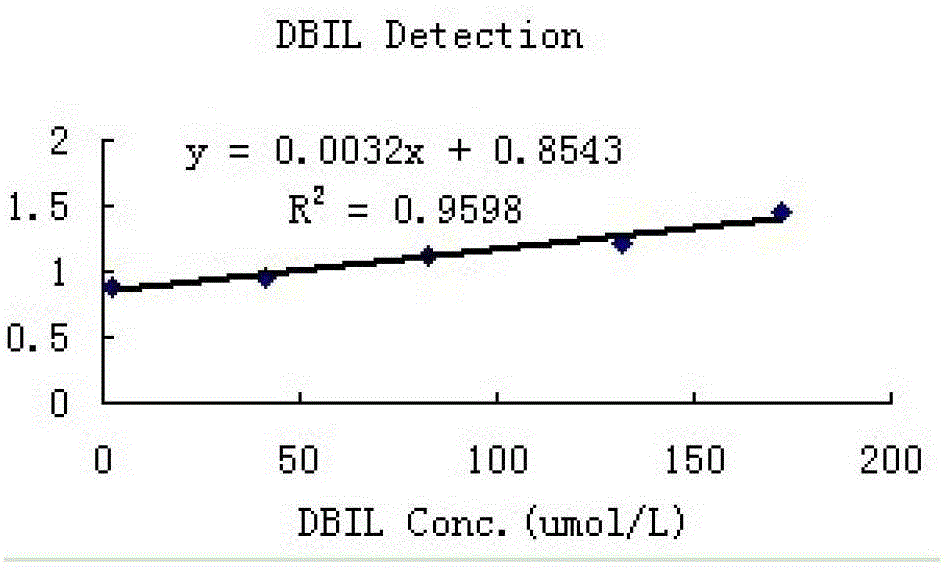
[0091] 2. Linear
[0092] The high-concentration serum samples collected from the hospital were diluted into different concentrations of analytes for detection, and the obtained DBIL linear results are shown in figure 1 .
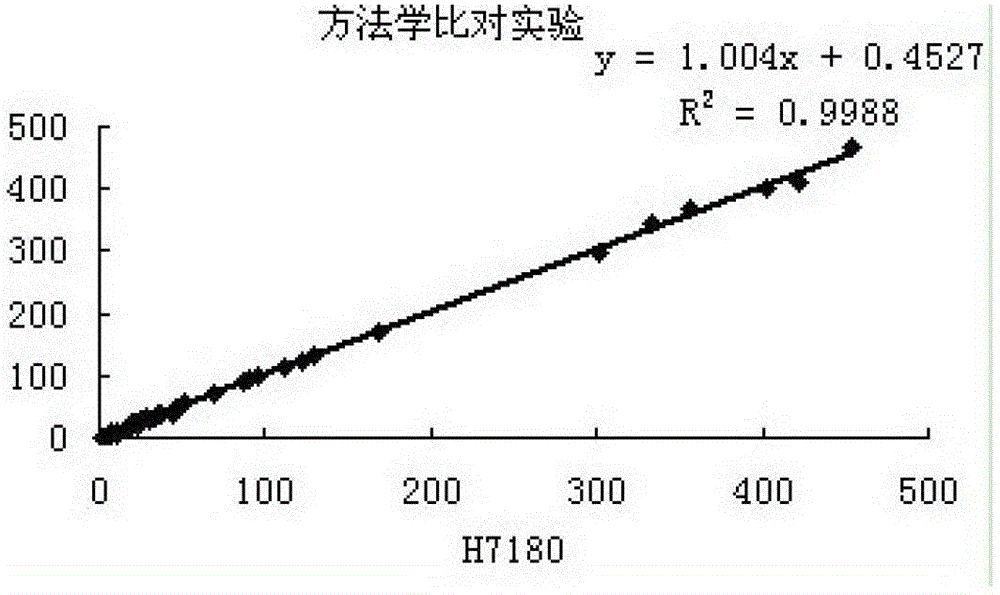
[0093] 3. Methodological comparison test ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com